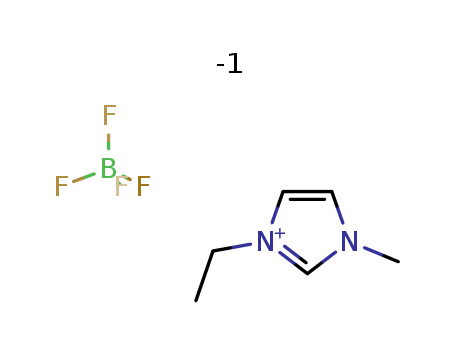
578-57-4
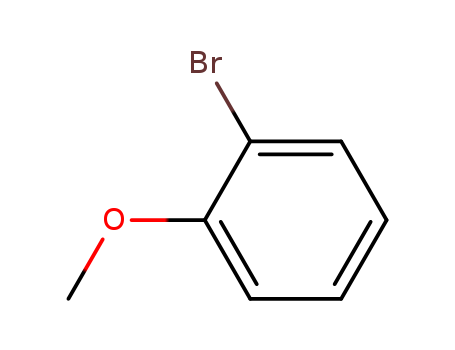
- Product Name:2-Bromoanisole
- Molecular Formula:C7H7BrO
- Purity:99%
- Molecular Weight:187.036
Product Details;
CasNo: 578-57-4
Molecular Formula: C7H7BrO
Appearance: colourless liquid
factory and supplier 578-57-4 2-Bromoanisole in stock
- Molecular Formula:C7H7BrO
- Molecular Weight:187.036
- Appearance/Colour:colourless liquid
- Vapor Pressure:0.21mmHg at 25°C
- Melting Point:2 °C(lit.)
- Refractive Index:n20/D 1.573(lit.)
- Boiling Point:215.999 °C at 760 mmHg
- Flash Point:96.667 °C
- PSA:9.23000
- Density:1.443 g/cm3
- LogP:2.45770
578-57-4 Relevant articles
Chain-Breaking Phenolic 2,3-Dihydrobenzo[b]selenophene Antioxidants: Proximity Effects and Regeneration Studies
Singh, Vijay P.,Yan, Jiajie,Poon, Jia-Fei,Gates, Paul J.,Butcher, Ray J.,Engman, Lars
, p. 15080 - 15088 (2017)
Phenolic 2,3-dihydrobenzo[b]selenophene ...
Direct bromodeboronation of arylboronic acids with CuBr2 in water
Tang, Yan-Ling,Xia, Xian-Song,Gao, Jin-Chun,Li, Min-Xin,Mao, Ze-Wei
supporting information, (2021/01/05)
An efficient and practical method has be...
Chiral secondary phosphine oxide pre-ligand and application thereof
-
Paragraph 0033-0036, (2021/10/20)
The invention discloses a chiral seconda...
Catalytic SNAr Hydroxylation and Alkoxylation of Aryl Fluorides
Kang, Qi-Kai,Li, Ke,Li, Yuntong,Lin, Yunzhi,Shi, Hang,Xu, Lun
supporting information, p. 20391 - 20399 (2021/08/13)
Nucleophilic aromatic substitution (SNAr...
Orthogonal Stability and Reactivity of Aryl Germanes Enables Rapid and Selective (Multi)Halogenations
Deckers, Kristina,Fricke, Christoph,Schoenebeck, Franziska
supporting information, p. 18717 - 18722 (2020/08/25)
While halogenation is of key importance ...
578-57-4 Process route
-
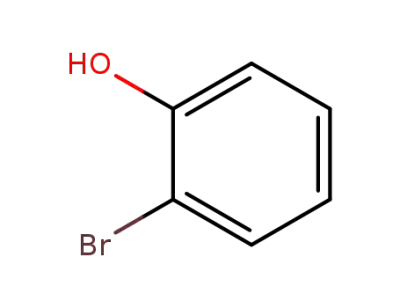
-
95-56-7
2-hydroxybromobenzene

-
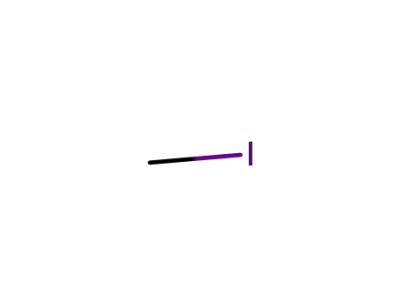
-
74-88-4
methyl iodide

-
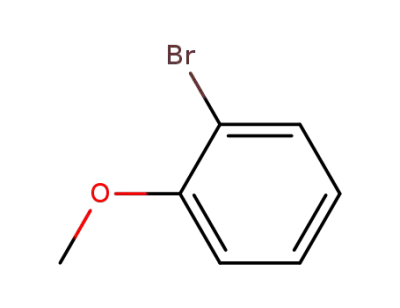
-
578-57-4
2-bromoanisole
| Conditions | Yield |
|---|---|
|
2-hydroxybromobenzene;
With
sodium hydride;
In
tetrahydrofuran; mineral oil;
at 0 ℃;
for 1.16667h;
Inert atmosphere;
methyl iodide;
In
tetrahydrofuran; mineral oil;
at 20 ℃;
for 19h;
Inert atmosphere;
Reflux;
|
97% |
|
With
potassium carbonate;
In
N,N-dimethyl-formamide;
at 20 ℃;
for 2.5h;
Inert atmosphere;
|
96% |
|
With
potassium carbonate;
In
acetonitrile;
at 81 ℃;
for 4h;
Inert atmosphere;
|
95% |
|
With
potassium hydroxide;
In
CD2Cl2; hexane; acetonitrile;
|
89% |
|
With
sodium hydroxide;
In
diethyl ether; ethanol;
|
|
|
With
potassium hydroxide;
In
ethanol; toluene;
|
|
|
With
potassium carbonate;
In
N,N-dimethyl-formamide;
at 20 ℃;
Inert atmosphere;
|
-
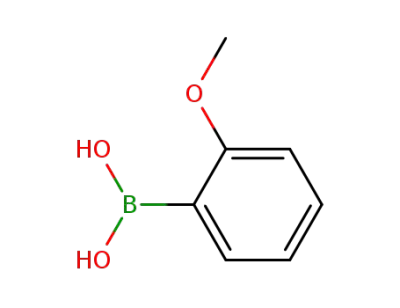
-
5720-06-9
2-Methoxyphenylboronic acid

-

-
578-57-4
2-bromoanisole
| Conditions | Yield |
|---|---|
|
With
N-Bromosuccinimide;
In
acetonitrile;
at 80 ℃;
for 12h;
|
97% |
|
With
tetrabutylammomium bromide; copper(ll) bromide;
In
water;
at 100 ℃;
for 8h;
Sealed tube;
|
82% |
|
With
1,10-Phenanthroline; oxygen; potassium bromide; copper(ll) bromide;
In
N,N-dimethyl-formamide;
at 130 ℃;
for 20h;
|
67% |
|
With
1,3-dibromo-5,5-dimethylimidazolidine-2,4-dione; sodium methylate;
In
methanol; water; acetonitrile;
at 23 ℃;
|
94 % Chromat. |
578-57-4 Upstream products
-
75-44-5
phosgene
-
60-29-7
diethyl ether
-
21702-84-1
2,4-dibromoanisole
-
95-56-7

2-hydroxybromobenzene
578-57-4 Downstream products
-
46719-55-5
4-(3-bromo-4-methoxyphenyl)-4-oxobutanoic acid
-
68204-81-9
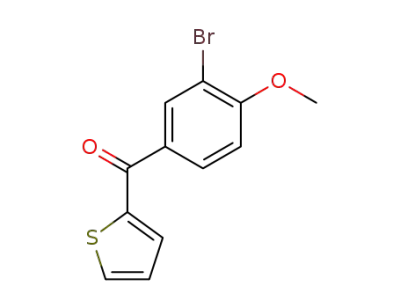
(3-bromo-4-methoxy-phenyl)-[2]thienyl ketone
-
579-75-9
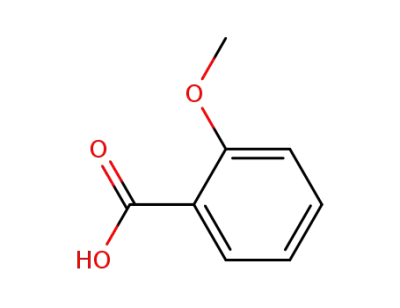
2-Methoxybenzoic acid
-
790-71-6
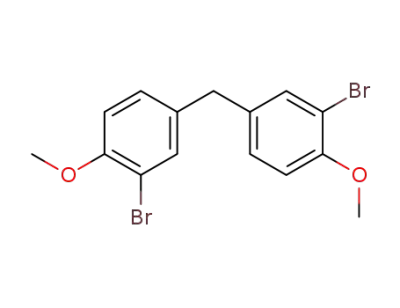
bis(3-bromo-4-methoxyphenyl)methane
Relevant Products
-
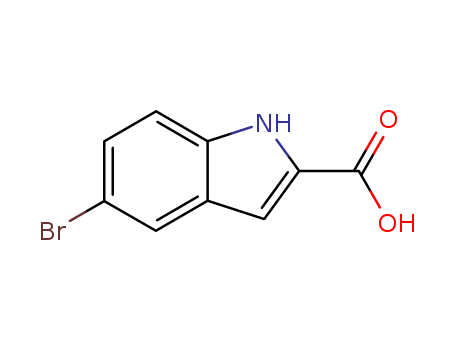
5-Bromoindole-2-carboxylic acid
CAS:7254-19-5
-
1-Ethyl-3-methylimidazolium tetrafluoroborate
CAS:143314-16-3
-
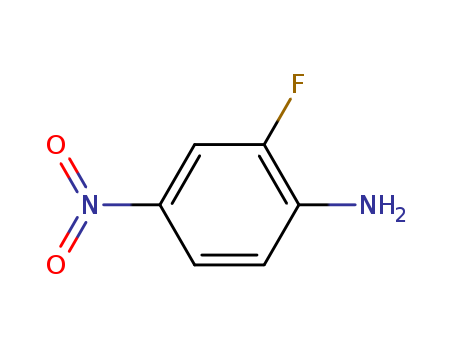
2-Fluoro-4-nitroaniline
CAS:369-35-7