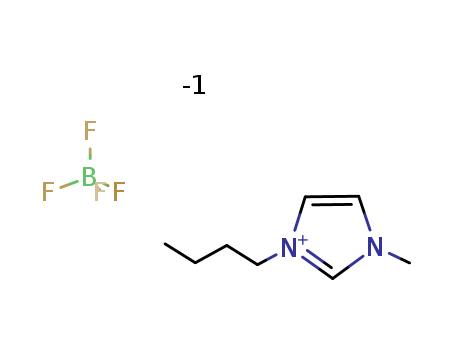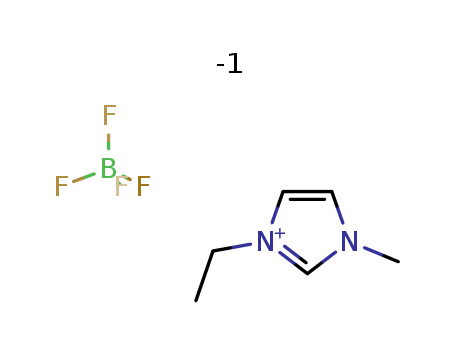
143314-16-3
- Product Name:1-Ethyl-3-methylimidazolium tetrafluoroborate
- Molecular Formula:C6H11N2.BF4
- Purity:99%
- Molecular Weight:197.971
Product Details;
CasNo: 143314-16-3
Molecular Formula: C6H11N2.BF4
factory and manufacture 143314-16-3 1-Ethyl-3-methylimidazolium tetrafluoroborate lonic liquid
- Molecular Formula:C6H11N2.BF4
- Molecular Weight:197.971
- Vapor Pressure:0-0Pa at 20-25℃
- Melting Point:15 °C(lit.)
- Refractive Index:n20/D 1.413(lit.)
- Boiling Point:>350 °C(lit.)
- Flash Point:>110 °C
- PSA:8.81000
- Density:1.294 g/mL at 25 °C(lit.)
- LogP:1.63250
1-Ethyl-3-methylimidazolium tetrafluoroborate(Cas 143314-16-3) Usage
|
Conductivity |
14.1 mS/cm (25 °C) |
|
General Description |
1-Ethyl-3-methylimidazolium tetrafluoroborate ([EMIM][BF4]) is an ionic liquid with notable electrochemical properties, including conductivity comparable to traditional salts like TEMABF4 when diluted in polar solvents such as propylene carbonate. It exhibits miscibility with organic solvents, enhancing conductivity in mixed systems. Additionally, its stability and electrochemical behavior are influenced by water, which can facilitate carbene formation and stabilization through hydrogen bonding. 1-Ethyl-3-methylimidazolium tetrafluoroborate is also synthesized with high purity for applications in electrolytes and other electrochemical processes. |
InChI:InChI=1/C6H11N2.BF4/c1-3-8-5-4-7(2)6-8;2-1(3,4)5/h4-6H,3H2,1-2H3;/q+1;-1
143314-16-3 Relevant articles
Physical and electrochemical properties of 1-alkyl-3-methylimidazolium tetrafluoroborate for electrolyte
Nishida, Tetsou,Tashiro, Yasutaka,Yamamoto, Masashi
, p. 135 - 141 (2003)
Three kinds of ionic liquids, 1-alkyl-3-...
Photoreduction of benzophenones by amines in room-temperature ionic liquids
Reynolds, John L.,Erdner, Kimberly R.,Jones, Paul B.
, p. 917 - 919 (2002)
(equation presented) The amine-mediated ...
Conductivities of binary mixtures of ionic liquids with polar solvents
Stoppa, Alexander,Hunger, Johannes,Buchner, Richard
, p. 472 - 479 (2009)
Data for the conductivity, κ, of selecte...
Synthesis of High-Purity Imidazolium Tetrafluoroborates and Bis(oxalato)borates
Schmitz, Paulo,Jakelski, Rene,Jalkanen, Kirsi,Winter, Martin,Bieker, Peter
, p. 2261 - 2264 (2017)
The synthesis and purification of imidaz...
Water-assisted stability of carbene: cyclic voltammetric investigation of 1-ethyl-3-methylimidazolium ethylsulfate ionic liquid
Jain, Preeti,Chaudhari, Vijay R.,Kumar, Anil
, p. 24126 - 24131 (2019)
In this work, we report electrochemical ...
Efficient absorption of ammonia with hydroxyl-functionalized ionic liquids
Li, Zhijie,Zhang, Xiangping,Dong, Haifeng,Zhang, Xiaochun,Gao, Hongshuai,Zhang, Suojiang,Li, Jianwei,Wang, Congmin
, p. 81362 - 81370 (2015)
Ammonia (NH3) emitted from the ammonia s...
Deciphering the anthelmintic activity of benzimidazolium salts by experimental and in-silico studies
Ranjan, Prabodh,Athar, Mohd,Vijayakrishna, Kari,Meena, Lalit K.,Vasita, Rajesh,Jha, Prakash C.
, p. 156 - 168 (2018)
Inspired from the facts that majority of...
Weakly-basic anion exchange resin scavenges impurities in ionic liquid synthesized from trialkyloxonium salt
Takao, Koichiro,Tsubomura, Taro
, p. 2497 - 2502,6 (2012)
A new purification method of ionic liqui...
Lewis Acid Catalyzed Synthesis of Cyanidoborates
Bl?sing, Kevin,Ellinger, Stefan,Harloff, J?rg,Schulz, Axel,Sievert, Katharina,T?schler, Christoph,Villinger, Alexander,Zur T?schler, Cornelia
, p. 1175 - 1183 (2016)
The reactions of [BF4]- salts with Me3Si...
Steady-state and time-resolved fluorescence behavior of C153 and PRODAN in room-temperature ionic liquids
Karmakar, Rana,Samanta, Anunay
, p. 6670 - 6675 (2002)
Room temperature ionic liquids have emer...
Interactions of CO2 with the homologous series of СnMIMBF4 ionic liquids studied in situ ATR-FTIR spectroscopy: spectral characteristics, thermodynamic parameters and their correlation
Adonin, Nikolai Y.,Kazarian, Sergei G.,Martyanov, Oleg N.,Nesterov, Nikolai S.,Prikhod'ko, Sergei A.,Shalygin, Anton S.
, (2020)
In this work, in situ ATR-FTIR spectrosc...
Improved preparation and use of room-temperature ionic liquids in lipase-catalyzed enantio- and regioselective acylations
Park,Kazlauskas
, p. 8395 - 8401 (2001)
Polar organic solvents such as methanol ...
Heteronuclear NOE spectroscopy of ionic liquids
Lingscheid, Yves,Arenz, Sven,Giernoth, Ralf
, p. 261 - 266 (2012)
19F,1H HOESY experiments with three ioni...
Greatly enhanced fluorescence of dicyanamide anion based ionic liquids confined into mesoporous silica gel
Zhang, Juan,Zhang, Qinghua,Shi, Feng,Zhang, Shiguo,Qiao, Botao,Liu, Lequan,Ma, Yubo,Deng, Youquan
, p. 229 - 234 (2008)
1-Ethyl-3-methylimidazolium dicyanamide ...
Correlation between hydrogen bond basicity and acetylene solubility in room temperature ionic liquids
Palgunadi, Jelliarko,Hong, Sung Yun,Lee, Jin Kyu,Lee, Hyunjoo,Lee, Sang Deuk,Cheong, Minserk,Kim, Hoon Sik
, p. 1067 - 1074 (2011)
Room temperature ionic liquids (RTILs) a...
Voltammetric study and electrodeposition of tellurium, lead, and lead telluride in room-temperature ionic liquid 1-ethyl-3-methylimidazolium tetrafluoroborate
Tsai, Ren-Wei,Hsieh, Yi-Ting,Chen, Po-Yu,Sun, I-Wen
, p. 49 - 56 (2014)
Room-temperature ionic liquid 1-ethyl-3-...
A novel method for preparation of imidazolium tetrafluoroborate ionic liquids
Egashira, Minato,Yamamoto, Yuji,Fukutake, Tsubasa,Yoshimoto, Nobuko,Morita, Masayuki
, p. 1261 - 1264 (2006)
A new method for the preparation of subs...
Lewis Acid Catalyzed Synthesis of Cyanidophosphates
Bl?sing, Kevin,Ellinger, Stefan,Harloff, J?rg,Schulz, Axel,Sievert, Katharina,T?schler, Christoph,Villinger, Alexander,Zurt?schler, Cornelia
, p. 4175 - 4188 (2016)
Salts containing new cyanido(fluorido)ph...
Alternative route to metal halide free ionic liquids
Takao, Koichiro,Ikeda, Yasuhisa
, p. 682 - 683 (2008)
An alternative synthetic route to metal ...
Self-assembly and lower critical solution temperature properties of supramolecular block copolymer/ionic liquid complexes depending on the alkyl chain length of imidazolium rings
Noh, Minjoo,Cho, Byoung-Ki
, p. 3587 - 3596 (2017)
We report the liquid crystalline (LC) as...
Separation of ethyl acetate and ethanol by room temperature ionic liquids with the tetrafluoroborate anion
Hu, Xuesheng,Li, Yingxia,Cui, Dannan,Chen, Biaohua
, p. 427 - 433 (2008)
Liquid-liquid equilibrium data are prese...
A quick and green approach to prepare [Rmim]OH and its application in hydrophilic ionic liquid synthesis
Gao, Jian,Liu, Jianguo,Li, Bo,Liu, Wenming,Xie, Yun,Xin, Yuchen,Yin, Ying,Jie, Xiao,Gu, Jun,Zou, Zhigang
, p. 1661 - 1666 (2011)
A quick and green process to prepare [Rm...
Four fluoroborates ion liquid synthetic method of the (by machine translation)
-
Page/Page column 9-10, (2019/05/22)
The invention discloses a four-tetrafluo...
Preparation method of high-purity supercapacitor electrolyte quaternary ammonium salt
-
Paragraph 0042-0044, (2018/08/28)
The invention provides a preparation met...
143314-16-3 Process route
-
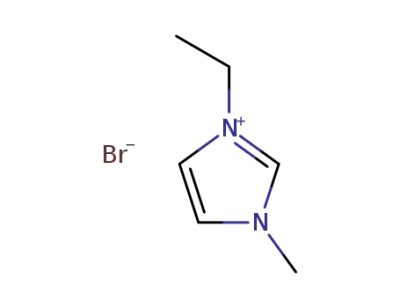
-
65039-08-9
3-ethyl-1-methyl-1H-imidazol-3-ium bromide

-
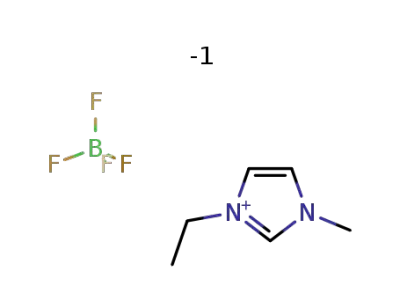
-
143314-16-3
1-ethyl-3-methylimidazolium tetrafluoroborate
| Conditions | Yield |
|---|---|
|
With
tetrafluoroboric acid; silver(l) oxide;
In
water;
at 20 ℃;
for 2h;
|
93% |
|
With
tetrafluoroboric acid; 1-hexene; dihydrogen peroxide;
at 25 ℃;
for 4h;
|
93% |
|
With
sodium tetrafluoroborate;
In
acetone;
for 12h;
|
50% |
|
With
tetrafluoroboric acid;
In
methanol;
|
|
|
With
sodium tetrafluoroborate;
In
acetone;
at 20 ℃;
|
|
|
With
sodium tetrafluoroborate;
In
acetone;
at 20 ℃;
for 24h;
|
|
|
3-ethyl-1-methyl-1H-imidazol-3-ium bromide;
With
sodium tetrafluoridobromate(III);
In
acetonitrile;
With
silver tetrafluoroborate;
In
methanol;
|
|
|
With
sodium tetrafluoroborate;
In
acetone;
at 20 ℃;
for 48h;
|
-
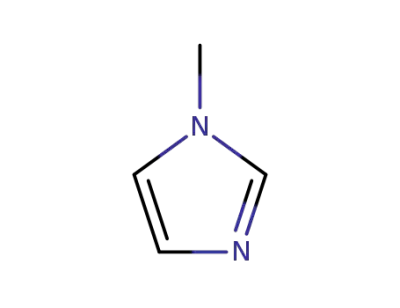
-
616-47-7
1-methyl-1H-imidazole

-
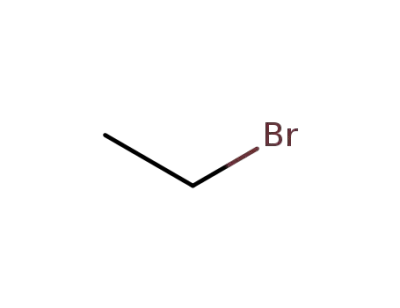
-
74-96-4
ethyl bromide

-
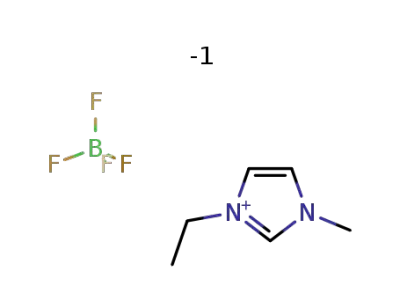
-
143314-16-3
1-ethyl-3-methylimidazolium tetrafluoroborate
| Conditions | Yield |
|---|---|
|
With
potassium tetrafluoroborate;
at 70 ℃;
for 4h;
|
94% |
|
With
sodium tetrafluoroborate;
at 70 ℃;
for 336h;
|
87% |
|
1-methyl-1H-imidazole; ethyl bromide;
In
1,1,1-trichloroethane;
at 80 ℃;
for 2h;
With
sodium tetrafluoroborate;
In
acetone;
at 20 ℃;
|
|
|
1-methyl-1H-imidazole; ethyl bromide;
at 64 ℃;
for 48h;
With
ammonium tetrafluroborate;
In
acetonitrile;
at 20 ℃;
for 48h;
Further stages.;
|
143314-16-3 Upstream products
-
65039-08-9

3-ethyl-1-methyl-1H-imidazol-3-ium bromide
-
616-47-7

1-methyl-1H-imidazole
-
74-96-4

ethyl bromide
-
65039-09-0
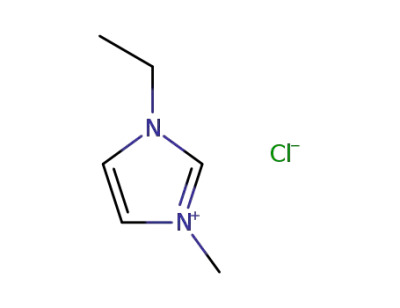
1-ethyl-3-methyl-1H-imidazol-3-ium chloride
143314-16-3 Downstream products
-
1207615-07-3
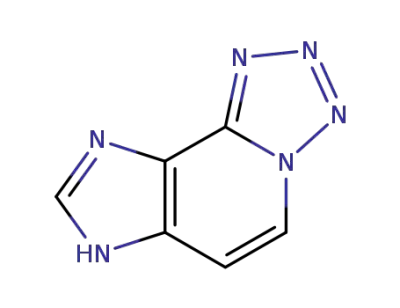
7H-imidazo[4,5-c]tetrazo[1,5-a]pyridine
-
377739-43-0
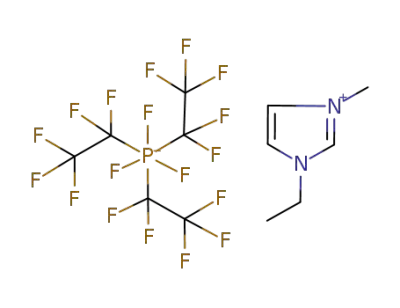
1-ethyl-3-methyl-imidazolium tris(pentafluoroethyl)trifluorophosphate
-
7637-07-2
boron trifluoride
-
60-29-7
diethyl ether
Relevant Products
-
1-Butyl-3-methylimidazolium tetrafluoroborate
CAS:174501-65-6
-
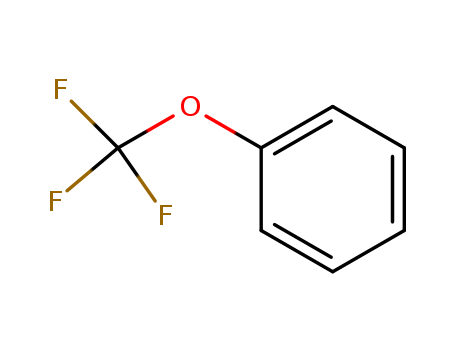
Trifluoromethoxybenzene
CAS:456-55-3
-
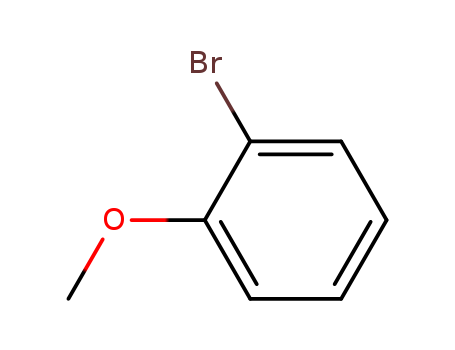
2-Bromoanisole
CAS:578-57-4