7254-19-5
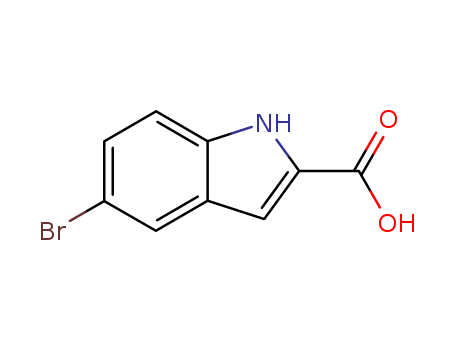
- Product Name:5-Bromoindole-2-carboxylic acid
- Molecular Formula:C9H6BrNO2
- Purity:99%
- Molecular Weight:240.056
Product Details;
CasNo: 7254-19-5
Molecular Formula: C9H6BrNO2
factory and supplier 7254-19-5 5-Bromoindole-2-carboxylic acid in stock
- Molecular Formula:C9H6BrNO2
- Molecular Weight:240.056
- Vapor Pressure:0mmHg at 25°C
- Melting Point:287-288 °C
- Refractive Index:1.749
- Boiling Point:470.932 °C at 760 mmHg
- PKA:4.25±0.30(Predicted)
- Flash Point:238.611 °C
- PSA:53.09000
- Density:1.828 g/cm3
- LogP:2.62860
5-Bromoindole-2-carboxylic acid(Cas 7254-19-5) Usage
InChI:InChI=1/C9H6BrNO2/c10-6-1-2-7-5(3-6)4-8(11-7)9(12)13/h1-4,11H,(H,12,13)
7254-19-5 Relevant articles
Synthesis, structure-activity relationship and antiviral activity of indole-containing inhibitors of Flavivirus NS2B-NS3 protease
Kneubehl, Alexander R.,Li, Xin,Lin, Yi-Lun,Nie, Shenyou,Rico-Hesse, Rebecca,Song, Yongcheng,Vogt, Megan B.,Wu, Fangrui,Wu, Xiaowei,Yao, Yuan,Zhao, Jidong
, (2021/08/26)
Zika virus belongs to the Flavivirus fam...
Substituted indole - 2 - formic acid (by machine translation)
-
Paragraph 0050; 0053, (2017/10/28)
This invention relates to a substituted ...
Cathepsin K inhibitor and application thereof
-
Paragraph 0219; 0220; 0221; 0276; 0277; 0278, (2017/08/28)
The invention relates to a cathepsin K i...
Synthesis of 3,3-Dihalo-2-oxindoles from 2-Substituted Indoles via Halogenation–Decarboxylation/Desulfonamidation–Oxidation Process
Jiang, Xiaojian,Zhang, Feng,Yang, Junjie,Yu, Pei,Yi, Peng,Sun, Yewei,Wang, Yuqiang
supporting information, p. 3938 - 3942 (2016/12/30)
A novel one-pot reaction which combines ...
7254-19-5 Process route
-
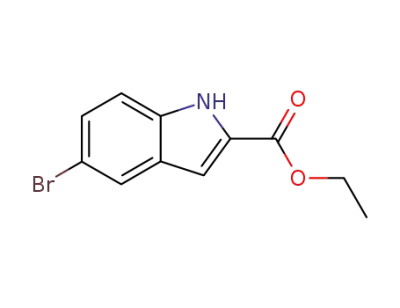
-
16732-70-0
ethyl 5-bromoindolecarboxylate

-
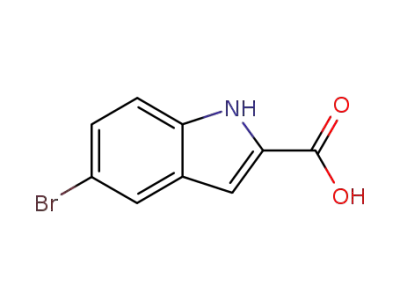
-
7254-19-5
5-bromo-2-indolecarboxylic acid
| Conditions | Yield |
|---|---|
|
With
sodium hydroxide;
at 110 ℃;
for 0.0333333h;
Microwave irradiation;
|
95% |
|
With
sodium hydroxide;
In
ethanol; water;
at 70 ℃;
for 2h;
|
92% |
|
ethyl 5-bromoindolecarboxylate;
With
sodium hydroxide;
In
methanol; water;
for 0.5h;
Reflux;
Green chemistry;
With
hydrogenchloride;
In
methanol; water;
at 40 ℃;
pH=3 - 4;
Green chemistry;
|
91% |
|
With
sodium hydroxide;
In
tetrahydrofuran; water;
at 50 ℃;
for 5h;
|
-
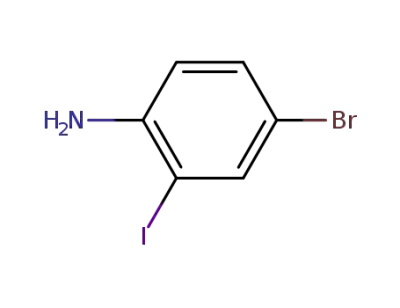
-
66416-72-6
4-bromo-2-iodoaniline

-
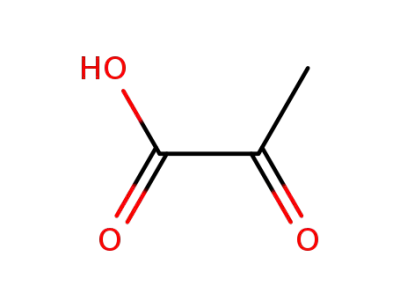
-
127-17-3
2-oxo-propionic acid

-

-
7254-19-5
5-bromo-2-indolecarboxylic acid
| Conditions | Yield |
|---|---|
|
With
1,4-diaza-bicyclo[2.2.2]octane; palladium diacetate;
In
N,N-dimethyl-formamide;
at 105 ℃;
for 4h;
|
7254-19-5 Upstream products
-
17403-17-7
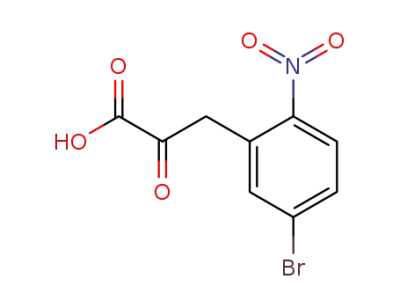
(5-bromo-2-nitro-phenyl)-pyruvic acid
-
66416-72-6

4-bromo-2-iodoaniline
-
127-17-3

2-oxo-propionic acid
-
16732-70-0

ethyl 5-bromoindolecarboxylate
7254-19-5 Downstream products
-
10075-50-0
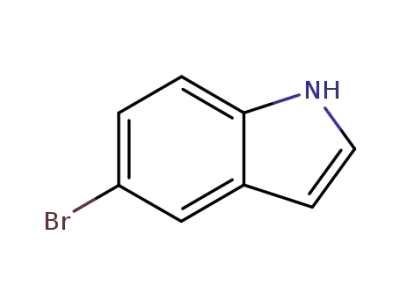
5-bromo-1H-indole
-
15861-24-2
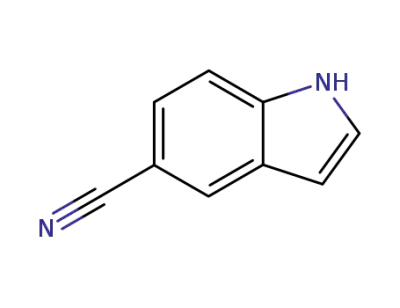
1H-indole-5-carbonitrile
-
210345-56-5
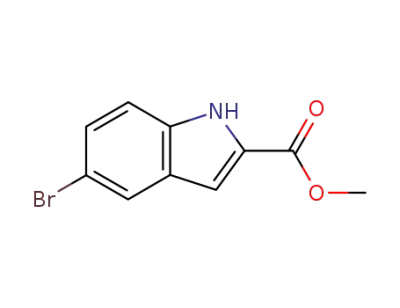
5-bromo-1H-indole-2-carboxylic acid methyl ester
-
889444-31-9
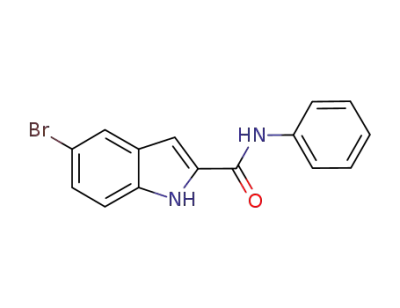
C15H11BrN2O
Relevant Products
-
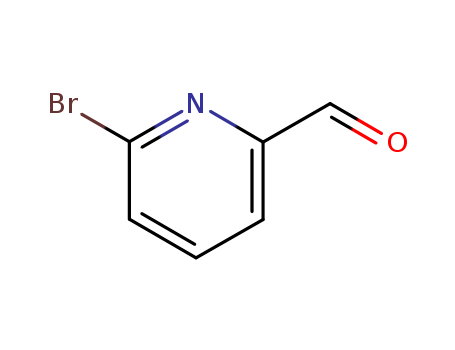
6-Bromopyridine-2-carbaldehyde
CAS:34160-40-2
-
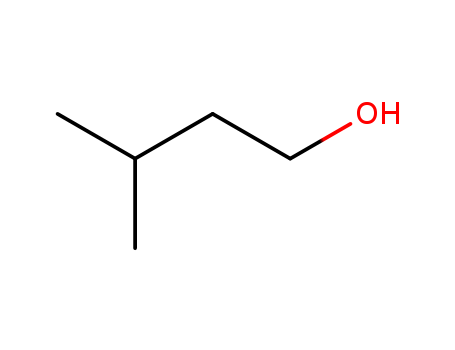
3-Methyl-1-butanol
CAS:123-51-3
-
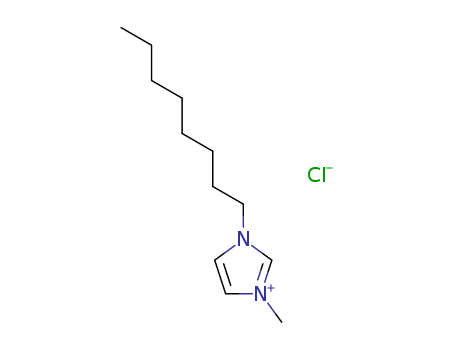
1-Methyl-3-octylimidazolium chloride
CAS:64697-40-1