25109-28-8
- Product Name:1-Bromo-4-Cyclohexylbenzene
- Molecular Formula:C12H15Br
- Purity:99%
- Molecular Weight:239.155
Product Details;
CasNo: 25109-28-8
Molecular Formula: C12H15Br
Appearance: Colorless to light yellow liquid
factory and supplier 25109-28-8 1-Bromo-4-Cyclohexylbenzene in stock
- Molecular Formula:C12H15Br
- Molecular Weight:239.155
- Appearance/Colour:Colorless to light yellow liquid
- Vapor Pressure:0.00366mmHg at 25°C
- Refractive Index:1.5595
- Boiling Point:290.172 °C at 760 mmHg
- Flash Point:127.852 °C
- PSA:0.00000
- Density:1.28 g/cm3
- LogP:4.49680
1-Bromo-4-cyclohexylbenzene(Cas 25109-28-8) Usage
InChI:InChI=1/C12H15Br/c13-12-8-6-11(7-9-12)10-4-2-1-3-5-10/h6-10H,1-5H2
25109-28-8 Relevant articles
Organic compound and, electronic component using same, and electronic device
-
Paragraph 0148-0152; 154, (2021/12/07)
The invention belongs to the field of or...
Organic compound, electronic device and electronic device (by machine translation)
-
Paragraph 0209-0210, (2020/04/22)
The present application relates to an or...
Ni-catalyzed Reductive Deaminative Arylation at sp3 Carbon Centers
Martin-Montero, Raul,Yatham, Veera Reddy,Yin, Hongfei,Davies, Jacob,Martin, Ruben
, p. 2947 - 2951 (2019/04/30)
A Ni-catalyzed reductive deaminative ary...
C?I-Selective Cross-Coupling Enabled by a Cationic Palladium Trimer
Diehl, Claudia J.,Scattolin, Thomas,Englert, Ulli,Schoenebeck, Franziska
supporting information, p. 211 - 215 (2018/12/13)
While there is a growing interest in har...
25109-28-8 Process route
-
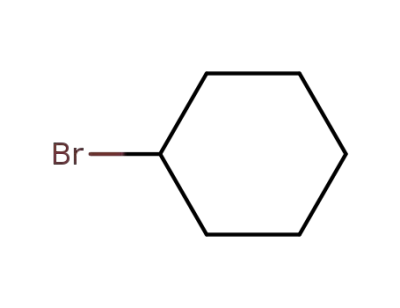
-
108-85-0
1-bromocyclohexane

-
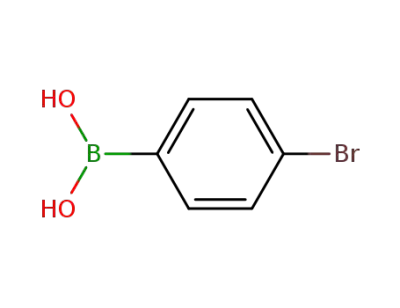
-
5467-74-3
4-Bromophenylboronic acid

-
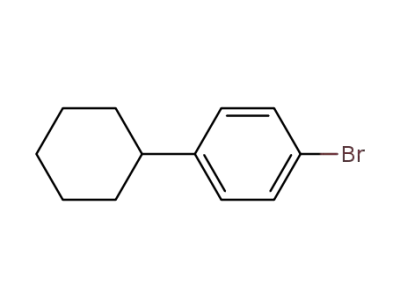
-
25109-28-8
4-bromo(cyclohexyl)benzene
| Conditions | Yield |
|---|---|
|
With
tetrakis(triphenylphosphine) palladium(0); tetrabutylammomium bromide; potassium carbonate;
In
water; toluene;
at 80 ℃;
for 4h;
Inert atmosphere;
|
87% |
-
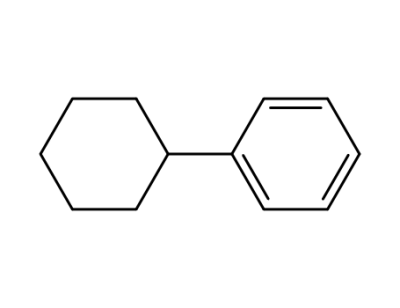
-
827-52-1,5379-26-0
1-phenyl-1-cyclohexane

-
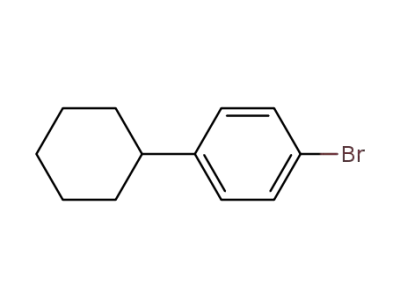
-
25109-28-8
4-bromo(cyclohexyl)benzene
| Conditions | Yield |
|---|---|
|
1-phenyl-1-cyclohexane;
With
ferric(III) bromide;
at 20 ℃;
for 0.166667h;
Inert atmosphere;
With
bromine;
at 0 - 20 ℃;
for 24h;
Inert atmosphere;
|
69% |
|
With
bromine; iodine;
|
|
|
With
bromine; iron;
|
|
|
With
bromine;
In
trifluoroacetic acid;
|
|
|
Multi-step reaction with 3 steps
1: durch Nitrierung
2: iron; diluted hydrochloric acid
3: Diazotization.Behandeln mit Kupfer(I)-bromid
With
hydrogenchloride; iron;
|
|
|
Multi-step reaction with 3 steps
1: nitric acid / 0 °C
2: iron; diluted hydrochloric acid
3: Diazotization.Behandeln mit Kupfer(I)-bromid
With
hydrogenchloride; nitric acid; iron;
|
|
|
Multi-step reaction with 3 steps
1: glacial acetic acid; nitric acid / 0 °C
2: iron; diluted hydrochloric acid
3: Diazotization.Behandeln mit Kupfer(I)-bromid
With
hydrogenchloride; nitric acid; iron; acetic acid;
|
25109-28-8 Upstream products
-
108-86-1
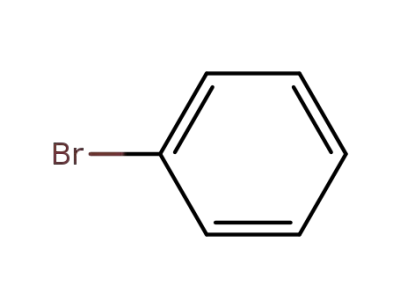
bromobenzene
-
542-18-7
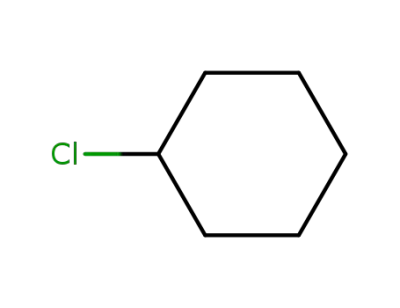
cyclohexyl chloride
-
827-52-1

1-phenyl-1-cyclohexane
-
6373-50-8
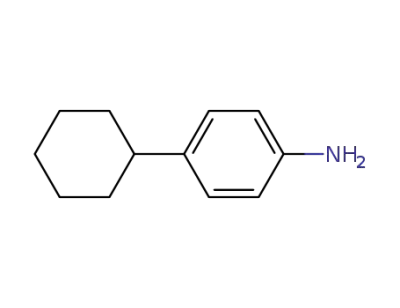
p-cyclohexylaniline
25109-28-8 Downstream products
-
81937-29-3
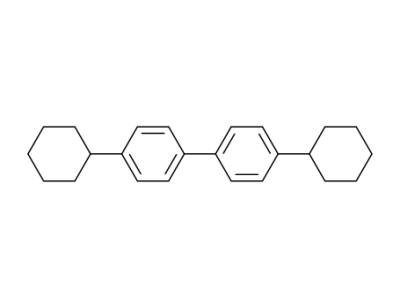
4,4'-Dicyclohexyl-biphenyl
-
57803-90-4
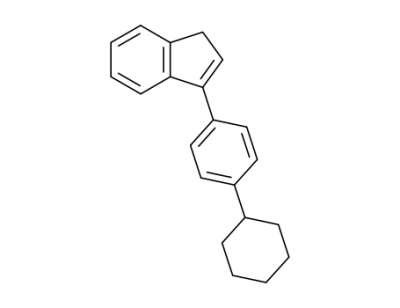
3-(4-Cyclohexyl-phenyl)-1H-indene
-
6728-90-1
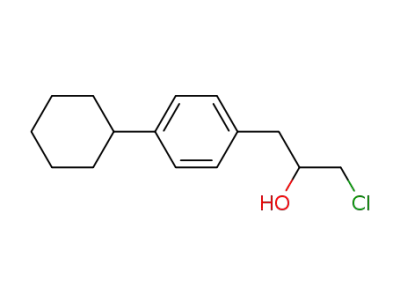
3-(p-Cyclohexyl-phenyl)-2-hydroxy-propylchlorid
-
87-82-1
hexabromobenzene
Relevant Products
-
5-Bromo-2-fluoro-m-xylene
CAS:99725-44-7
-
Decafluorbiphenyl
CAS:434-90-2
-
D-Alaninol
CAS:35320-23-1