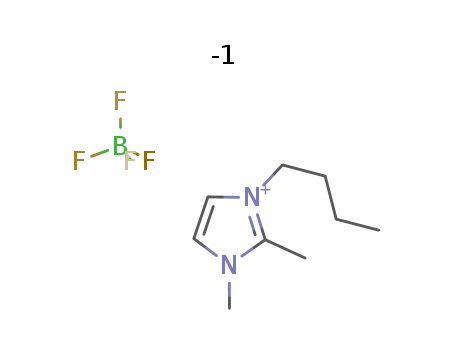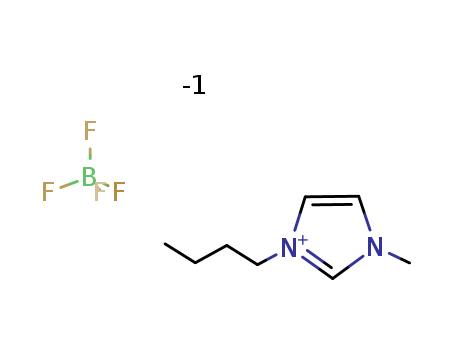
174501-65-6
- Product Name:1-Butyl-3-methylimidazolium tetrafluoroborate
- Molecular Formula:C8H15N2.BF4
- Purity:99%
- Molecular Weight:226.025
Product Details;
CasNo: 174501-65-6
Molecular Formula: C8H15N2.BF4
Appearance: Clear yellowish-orange oil
factory and manufacture 174501-65-6 1-Butyl-3-methylimidazolium tetrafluoroborate lonic liquid
- Molecular Formula:C8H15N2.BF4
- Molecular Weight:226.025
- Appearance/Colour:Clear yellowish-orange oil
- Melting Point:-71 °C
- Refractive Index:n20/D 1.52
- Flash Point:288 °C
- PSA:8.81000
- Density:1.21 g/mL at 20 °C(lit.)
- LogP:2.41270
1-Butyl-3-methylimidazolium tetrafluoroborate(Cas 174501-65-6) Usage
|
solubility |
Miscible with acetone, acetonitrile, ethyl acetate, isopropyl alcohol and methylene chloride. Immiscible with hexane, toluene and water. |
|
description |
1-Butyl-3-methylimidazolium hexafluorophosphate, also known as BMIM-PF6, is a viscous, colourless, hydrophobic and non-water-soluble ionic liquid with a melting point[1] of -8 °C. Together with 1-butyl-3-methylimidazolium tetrafluoroborate, BMIM-BF4, it is one of the most widely studied ionic liquids. It is known to very slowly decompose in the presence of water. |
|
Physical properties |
Clear yellowish-orange oil |
|
General Description |
1-Butyl-3-methylimidazolium tetrafluoroborate is a high-purity ionic liquid (HPIL) with low water and halogen content. |
InChI:InChI=1/C8H15N2.BF4/c1-3-4-5-10-7-6-9(2)8-10;2-1(3,4)5/h6-8H,3-5H2,1-2H3;/q+1;-1
174501-65-6 Relevant articles
Volumetric properties of 1-butyl-3-methylimidazolium tetrafluoroborate- glucose-water systems
Jin, Hui X.,Chen, Han Y.
, p. 1134 - 1138 (2012)
Densities for 1-butyl-3-methylimidazoliu...
Electrochemistry of 1-butyl-3-methyl-1H-imidazolium tetrafluoroborate ionic liquid
Xiao, Li,Johnson, Keith E.
, p. E307-E311 (2003)
The electrochemistry of 1-butyl-3-methyl...
Crystallographic Insights into the Behavior of Highly Acidic Metal Cations in Ionic Liquids from Reactions of Titanium Tetrachloride with [1-Butyl-3-Methylimidazolium][X] Ionic Liquids (X = Chloride, Bromide, Tetrafluoroborate)
Mishra, Manish Kumar,Kelley, Steven P.,Dilip, Meghna,Vaid, Thomas P.,Cordes, David B.,Griffin, Scott T.,Rogers, Robin D.
, p. 1764 - 1773 (2019)
Highly charged metal ions are difficult ...
A study of halide nucleophilicity in ionic liquids
Lancaster,Welton,Young
, p. 2267 - 2270 (2001)
The relative nucleophilicity of chloride...
Interaction between the ionic liquids 1-alkyl-3-methylimidazolium tetrafluoroborate and Pluronic P103 in aqueous solution: A DLS, SANS and NMR study
Parmar, A.,Bahadur, P.,Aswal, V. K.
, p. 137 - 143,7 (2012)
The effect of three ionic liquids (ILs) ...
Determination of residual chloride content in ionic liquids using LA-ICP-MS
Bonta, Maximilian,Anderl, Thomas,Cognigni, Alice,Hejazifar, Mahtab,Bica, Katharina,Limbeck, Andreas
, p. 90273 - 90279 (2016)
Nowadays, ionic liquids (ILs) are used i...
Thermodynamics of cesium complexes formation with 18-crown-6 in ionic liquids
Vendilo,Roenkkoemaeki,Hannu-Kuure,Lajunen,Asikkala,Krasovsky,Chernikova,Oksman,Lajunen,Tuomi,Popov
, p. 223 - 230 (2010)
Thermodynamic data for cesium complexes ...
A silver and water free metathesis reaction: A route to ionic liquids
Srour, Hassan,Rouault, Helene,Santini, Catherine C.,Chauvin, Yves
, p. 1341 - 1347 (2013)
A versatile, cheaper, silver and water-f...
A facile and efficient route to hydrophilic ionic liquids through metathesis reaction performed in saturated aqueous solution
Chen, Zhengjian,Li, Zuopeng,Ma, Xiaoyun,Long, Panfeng,Zhou, Yun,Xu, Lin,Zhang, Shiguo
, p. 1303 - 1307 (2017)
The preparation of ionic liquids most of...
Comparative Investigation of the Ionicity of Aprotic and Protic Ionic Liquids in Molecular Solvents by using Conductometry and NMR Spectroscopy
Thawarkar, Sachin,Khupse, Nageshwar D.,Kumar, Anil
, p. 1006 - 1017 (2016)
Electrical conductivity (σ), viscosity (...
Preparation of 1-butyl-3-methylimidazolium salts and study of their phase behavior and intramolecular intractions
Gruzdev,Ramenskaya,Chervonova,Kumeev
, p. 1720 - 1727 (2009)
Seven organic salts of 1-butyl-3-methyli...
Synthesis of Metal Nanoparticles and Metal Fluoride Nanoparticles from Metal Amidinate Precursors in 1-Butyl-3-Methylimidazolium Ionic Liquids and Propylene Carbonate
Schütte, Kai,Barthel, Juri,Endres, Manuel,Siebels, Marvin,Smarsly, Bernd M.,Yue, Junpei,Janiak, Christoph
, p. 137 - 148 (2017)
Decomposition of transition-metal amidin...
Water effect on physicochemical properties of 1-butyl-3-methylimidazolium based ionic liquids with inorganic anions
Grishina,Ramenskaya,Gruzdev,Kraeva
, p. 267 - 272 (2013)
Room temperature ionic liquids of 1-buty...
Extractions of Alkaloids Codeine and Caffeine with [Bmim][BF4]/Carbohydrate Aqueous Biphasic Systems as a Novel Class of Liquid-Liquid Extraction Systems
Jamehbozorg, Bahman,Sadeghi, Rahmat
, p. 916 - 925 (2019)
Liquid-liquid equilibrium (LLE) data for...
Microwave assisted Suzuki reaction in N-butylpyridinium salts/water systems
Dos Santos Castro, Kelly L.,De Lima, Paulo G.,E Miranda, Leandro S.M.,De Souza, Rodrigo O.M.A.
, p. 4168 - 4171 (2011)
A solvent mixture containing the ionic l...
Green chemistry with a novel 5.8-GHz microwave apparatus. Prompt one-pot solvent-free synthesis of a major ionic liquid: The 1-butyl-3-methylimidazolium tetrafluoroborate system
Horikoshi, Satoshi,Hamamura, Tomofumi,Kajitani, Masatsugu,Yoshizawa-Fujita, Masahiro,Serpone, Nick
, p. 1089 - 1093 (2008)
This article reports for the first time ...
Effects of methylation at the 2 position of the cation ring on phase behaviors and conformational structures of imidazolium-based ionic liquids
Endo, Takatsugu,Kato, Tatsuya,Nishikawa, Keiko
, p. 9201 - 9208 (2010)
The proton at the 2 position of the cati...
Aerobic and electrochemical oxidative cross-dehydrogenative-coupling (CDC) reaction in an imidazolium-based ionic liquid
Basle, Olivier,Borduas, Nadine,Dubois, Pauline,Chapuzet, Jean Marc,Chan, Tak-Hang,Lessard, Jean,Li, Chao-Jun
, p. 8162 - 8166 (2010)
The ionic liquid 1-butyl-3methylimidazol...
An improved preparation of 1,3-dialkylimidazolium tetrafluoroborate ionic liquids using microwaves
Namboodiri, Vasudevan V,Varma, Rajender S
, p. 5381 - 5383 (2002)
An efficient microwave protocol is descr...
Lewis basic ionic liquids-catalyzed conversion of carbon dioxide to cyclic carbonates
Yang, Zhen-Zhen,He, Liang-Nian,Miao, Cheng-Xia,Chanfreau, Sebastien
, p. 2233 - 2240 (2010)
A series of easily prepared Lewis basic ...
Activity coefficients at infinite dilution of organic compounds in 1-butyl-3-methylimidazolium tetrafluoroborate using inverse gas chromatography
Revelli, Anne-Laure,Mutetet, Fabrice,Turmine, Mireille,Solimando, Roland,Jaubert, Jean-Noel
, p. 90 - 101 (2009)
Activity coefficients at infinite diluti...
Vapor Pressure Osmometry Studies of Aqueous Ionic Liquid-Carbohydrate Systems
Jamehbozorg, Bahman,Sadeghi, Rahmat
, p. 331 - 340 (2018)
Precise vapor pressure osmometry (VPO) m...
Synthesis of symmetrical and unsymmetrical 3,3-di(indolyl)indolin-2-ones under controlled catalysis of ionic liquids
Rad-Moghadam, Kurosh,Sharifi-Kiasaraie, Masoumeh,Taheri-Amlashi, Homayun
, p. 2316 - 2321 (2010)
Three ionic liquids, [BMIM][BF4] doped w...
Efficient and chromaticity stable green and white organic light-emitting devices with organic-inorganic hybrid materials
Thanikachalam, Venugopal,Seransenguttuvan, Balu,Jayabharathi, Jayaraman
, p. 21206 - 21221 (2020)
Efficient inverted bottom emissive organ...
Ionic liquid-assisted gelation of an organic solvent
Smith, Nicholas W.,Knowles, Joshua,Albright, John G.,Dzyuba, Sergei V.
, p. 83 - 87 (2010)
Tetrafluoroborate-containing ionic liqui...
Volumetric Properties of Aqueous Ionic-Liquid Solutions at Different Temperatures
Shekaari, Hemayat,Zafarani-Moattar, Mohammed Taghi,Kazempour, Amir,Ghasedi-Khajeh, Zakiyeh
, p. 1750 - 1755 (2015)
Density measurements were carried out fo...
Study of a novel gel electrolyte based on poly-(methoxy/hexadecyl- poly(ethylene glycol) methacrylate) co-polymer plasticized with 1-butyl-3-methylimidazolium tetrafluoroborate
Wang, Long,Zhu, Hua-Jun,Zhai, Wei,Cai, Feng,Liu, Xiao-Min,Yang, Hui
, p. 36357 - 36365 (2014)
Compared with traditional liquid electro...
Liquid Phase Behavior of Imidazolium-Based Ionic Liquids with Alcohols
Crosthwaite, Jacob M.,Aki, Sudhir N. V. K.,Maginn, Edward J.,Brennecke, Joan F.
, p. 5113 - 5119 (2004)
A systematic study of the impact of diff...
Ultrasound-assisted surfactant/ionic liquid aqueous two-phase system extraction prior to high performance liquid chromatography for the determination of tetracyclines in milk and honey samples
Antep, Hayriye Mine,Mumcu, Ta?k?n,Bostanci, Kamil,Seyhan Bozkurt, Serap,Merdivan, Melek
, p. 955 - 966 (2017)
In this work, an ultrasonic-assisted sur...
Efficient synthesis of 1,3-dialkylimidazolium-based ionic liquids: The modified continuous Radziszewski reaction in a microreactor setup
Zimmermann, Johannes,Ondruschka, Bernd,Stark, Annegret
, p. 1102 - 1109 (2010)
By making use of a modified Radziszewski...
PET depolymerisation in supercritical ethanol catalysed by [Bmim][BF 4]
Nunes, Catia Santos,Vieira Da Silva, Michael Jackson,Cristina Da Silva, Danielle,Freitas, Adonilson Dos Reis,Rosa, Fernanda Andreia,Rubira, Adley Forti,Muniz, Edvani Curti
, p. 20308 - 20316 (2014)
Poly(ethylene terephthalate) (PET) was s...
β-Diimine-methallyl nickel complexes in ionic liquid: A biphasic green system for the high selective styrene dimerization
Landolsi, Kamel,Msaddek, Moncef
, (2022/02/23)
The selective head-to-tail dimerization ...
Convenient synthesis of long alkyl-chain triazolylglycosides using ionic liquid as dual promoter-solvent: Readily access to non-ionic triazolylglycoside surfactants for evaluation of cytotoxic activity
Ketsomboon, Nutthanicha,Saeeng, Rungnapha,Sirion, Uthaiwan,Srisook, Klaokwan
, (2021/08/26)
A convenient method for the one-pot synt...
Validation of structural grounds for anomalous molecular mobility in ionic liquid glasses
Adonin, Nicolay Yu.,Bakulina, Olga D.,Fedin, Matvey V.,Ivanov, Mikhail Yu.,Kiryutin, Alexey S.,Prikhod’ko, Sergey A.
supporting information, (2021/10/01)
Ionic liquid (IL) glasses have recently ...
Quantum dots in which ionic liquids are ion-bonded and their preparation method
-
Page/Page column 15, (2021/09/01)
A quantum dot particle in which an ionic...
174501-65-6 Process route
-

-
85100-77-2
1-n-butyl-3-methylimidazolim bromide

-
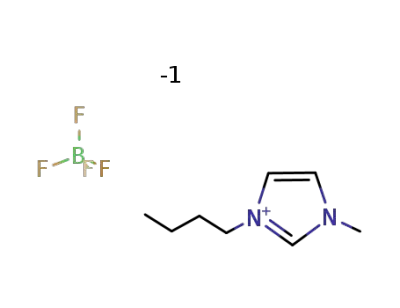
-
174501-65-6
1-butyl-3-methylimidazolium Tetrafluoroborate
| Conditions | Yield |
|---|---|
|
With
ammonium tetrafluoroborate;
In
water;
at 20 ℃;
for 2h;
|
100% |
|
With
sodium tetrafluoroborate;
In
acetone;
at 20 ℃;
for 24h;
|
98% |
|
With
sodium tetrafluoroborate;
In
acetone;
for 48h;
Inert atmosphere;
Darkness;
|
95% |
|
With
sodium tetrafluoroborate;
In
acetone;
at 20 ℃;
for 24h;
Inert atmosphere;
|
95% |
|
With
tetrafluoroboric acid; 1-hexene; dihydrogen peroxide;
at 25 ℃;
for 3h;
|
93% |
|
With
ammonium tetrafluroborate;
at 80 - 110 ℃;
for 0.0416667h;
Irradiation;
microwave;
|
92% |
|
With
silver tetrafluoroborate;
for 2h;
Darkness;
|
87% |
|
With
tetrafluoroboric acid; silver(l) oxide;
In
water;
at 20 ℃;
for 2h;
|
87% |
|
With
sodium tetrafluoroborate;
In
dichloromethane;
for 48h;
|
82% |
|
With
tetrafluoroboric acid;
In
water;
for 24h;
|
59% |
|
With
tetrafluoroboric acid;
In
methanol;
|
|
|
With
ammonium tetrafluroborate;
In
water;
for 24h;
|
1.6 mmol |
|
With
sodium tetrafluoroborate;
|
|
|
With
sodium tetrafluoroborate;
In
water;
at 20 ℃;
|
|
|
With
sodium tetrafluoroborate;
In
acetone;
at 20 ℃;
for 96h;
|
|
|
With
sodium tetrafluoroborate;
at 100 ℃;
for 0.25h;
Microwave irradiation;
Neat (no solvent);
|
|
|
With
sodium tetrafluoroborate;
In
acetone;
at 20 ℃;
for 48h;
|
|
|
With
sodium tetrafluoroborate;
In
acetonitrile;
at 20 ℃;
for 72h;
|
|
|
With
sodium tetrafluoroborate;
In
acetone;
at 20 ℃;
for 24h;
|
|
|
With
sodium tetrafluoroborate;
In
acetone;
at 20 ℃;
for 24h;
|
|
|
With
sodium tetrafluoroborate;
In
acetone;
|
|
|
With
sodium tetrafluoroborate;
In
propan-1-ol;
for 24h;
|
|
|
With
sodium tetrafluoroborate;
In
methanol;
|
|
|
With
sodium tetrafluoroborate;
at 20 ℃;
for 6h;
|
|
|
With
sodium tetrafluoroborate;
In
acetone;
at 20 ℃;
for 24h;
|
|
|
With
ammonium tetrafluoroborate;
In
acetonitrile;
for 24h;
Reflux;
|
|
|
With
sodium tetrafluoroborate;
In
water;
|
|
|
With
sodium tetrafluoroborate;
|
-

-
13755-29-8
sodium tetrafluoroborate

-
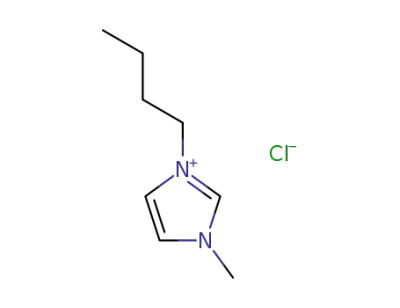
-
79917-90-1
1-butyl-3-methylimidazolium chloride

-
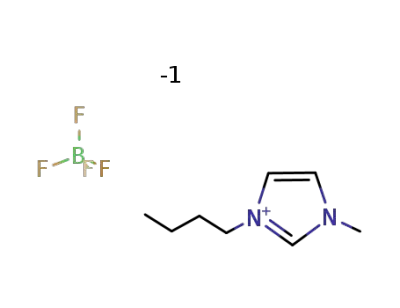
-
174501-65-6
1-butyl-3-methylimidazolium Tetrafluoroborate
| Conditions | Yield |
|---|---|
|
In
water;
Green chemistry;
|
99.2% |
|
In
acetone;
react. of equal moles of (C3H3N2CH3C4H9)Cl with NaBF4 in acetone; stirring at room temp. for 24 h; filtering and vac. drying overnight at 50°C; detd. by NMR;
|
90% |
|
In
water;
at 14 ℃;
|
89% |
|
In
acetone;
at 20 ℃;
for 18h;
|
89% |
|
In
acetone;
at 20 ℃;
for 48h;
|
86.7% |
|
In
acetone;
at 20 ℃;
for 48h;
|
85.1% |
|
In
acetone;
at 20 ℃;
for 48h;
|
85.4% |
|
In
water;
at 25 - 80 ℃;
for 10.5h;
under 0.000750075 Torr;
|
80% |
|
In
acetone;
at 20 ℃;
for 16h;
|
80% |
|
In
acetone;
at 20 ℃;
for 40h;
|
58% |
|
In
dichloromethane;
at 20 ℃;
for 24h;
Inert atmosphere;
|
52.3% |
|
In
water;
for 24h;
|
|
|
In
dichloromethane;
1.2 equiv. amt. of Na salt reacted with org. compd. in CH2Cl2; filtered, extd. with H2O to remove unreacted org. compd.;
|
|
|
In
water;
at 20 ℃;
for 3h;
|
|
|
In
acetone;
at 89.84 ℃;
for 4h;
|
|
|
In
water;
|
|
|
In
acetone;
at 50 ℃;
for 6h;
|
174501-65-6 Upstream products
-
79917-90-1

1-butyl-3-methylimidazolium chloride
-
616-47-7
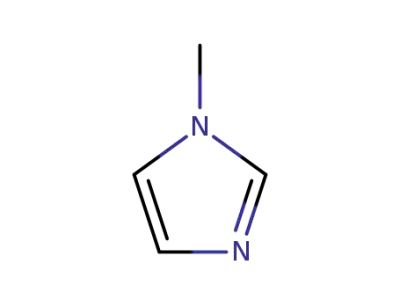
1-methyl-1H-imidazole
-
50-00-0
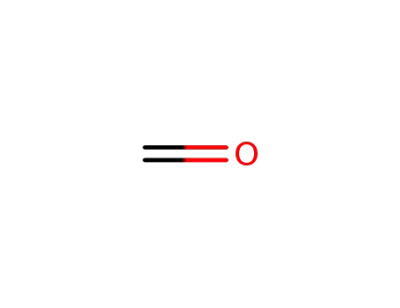
formaldehyd
-
131543-46-9
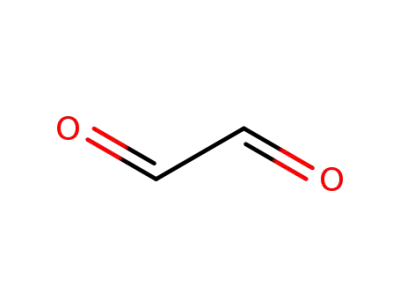
Glyoxal
174501-65-6 Downstream products
-
4316-42-1
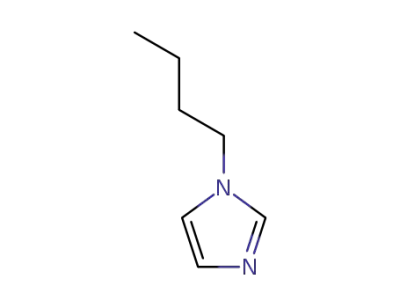
1-Butylimidazole
-
1126-80-3
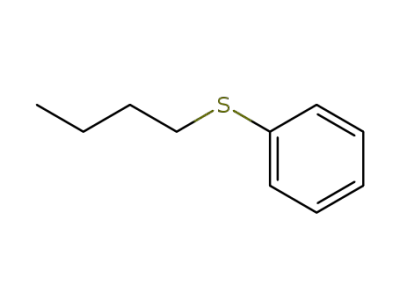
(n-butylthio)benzene
-
100-68-5
methyl-phenyl-thioether
-
56056-49-6

(n-dodecylthio)benzene
Relevant Products
-
1-butyl-2,3-dimethylimidazolium tetrafluoroborate
CAS:402846-78-0
-
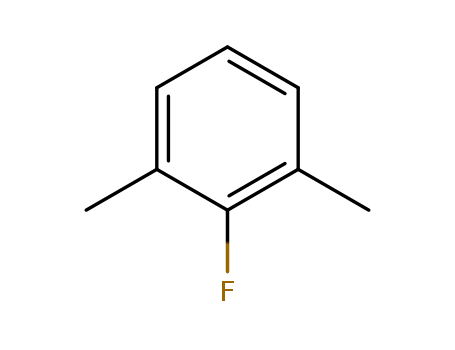
2,6-Dimethylfluorobenzene
CAS:443-88-9
-
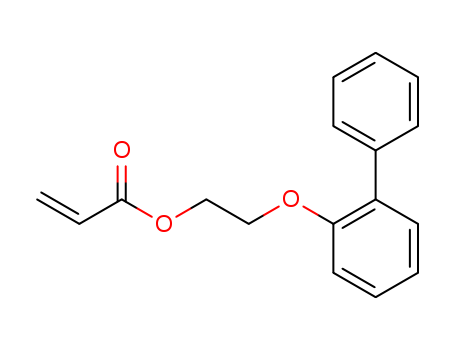
2-Propenoic acid 2-([1,1'-biphenyl]-2-yloxy)ethyl ester
CAS:91442-24-9