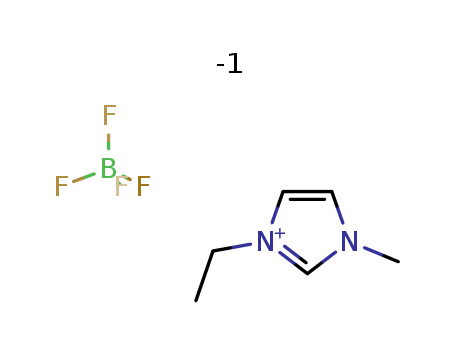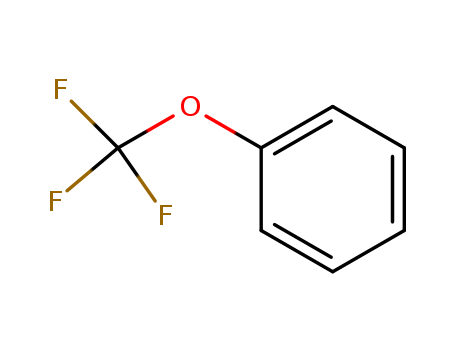
456-55-3
- Product Name:Trifluoromethoxybenzene
- Molecular Formula:C7H5F3O
- Purity:99%
- Molecular Weight:162.111
Product Details;
CasNo: 456-55-3
Molecular Formula: C7H5F3O
Appearance: Colorless to light yellow liquid
factory and supplier 456-55-3 Trifluoromethoxybenzene in stock
- Molecular Formula:C7H5F3O
- Molecular Weight:162.111
- Appearance/Colour:Colorless to light yellow liquid
- Vapor Pressure:41.3 mm Hg ( 25 °C)
- Melting Point:-50oC
- Refractive Index:n20/D 1.406(lit.)
- Boiling Point:102.8 °C at 760 mmHg
- Flash Point:12.2 °C
- PSA:9.23000
- Density:1.248 g/cm3
- LogP:2.58520
(Trifluoromethoxy)benzene(Cas 456-55-3) Usage
|
Synthesis Reference(s) |
The Journal of Organic Chemistry, 29, p. 1, 1964 DOI: 10.1021/jo01024a001 |
|
General Description |
(Trifluoromethoxy)benzene is an aryl trifluoromethyl ether. (Trifluoromethoxy)benzene can be prepared from 4-chloro-1-(trifluoromethoxy)benzene via hydrogenolysis. Conformation of (trifluoromethoxy)benzene in the gas phase has been studied by electron diffraction and spectroscopy supplemented with ab initio calculations. |
InChI:InChI=1/C7H5F3O/c8-7(9,10)11-6-4-2-1-3-5-6/h1-5H
456-55-3 Relevant articles
-
Allison,Cady
, p. 1089 (1959)
-
Synthesis conditions and activity of various Lewis acids for the fluorination of trichloromethoxy-benzene by HF in liquid phase
Salomé,Mauger,Brunet,Schanen
, p. 1947 - 1950 (2004)
The experimental conditions (temperature...
Radical Hydrodehalogenation of Aryl Halides with H2 Catalyzed by a Phenanthroline-Based PNNP Cobalt(I) Complex
Iizuka, Kosuke,Ishizaka, Yusuke,Jheng, Nai-Yuan,Minami, Yasunori,Naganawa, Yuki,Nakajima, Yumiko,Sekiguchi, Akira
, p. 2320 - 2329 (2022/02/16)
Radical hydrodehalogenation of aryl hali...
Radical C?H Trifluoromethoxylation of (Hetero)arenes with Bis(trifluoromethyl)peroxide
Dix, Stefan,Golz, Paul,Schmid, Jonas R.,Riedel, Sebastian,Hopkinson, Matthew N.
supporting information, p. 11554 - 11558 (2021/07/09)
Trifluoromethoxylated (hetero)arenes are...
Direct Trifluoromethylation of Alcohols Using a Hypervalent Iodosulfoximine Reagent
Kalim, Jorna,Duhail, Thibaut,Pietrasiak, Ewa,Anselmi, Elsa,Magnier, Emmanuel,Togni, Antonio
supporting information, p. 2638 - 2642 (2021/01/21)
The direct trifluoromethylation of a var...
Electrochemical Activation of Diverse Conventional Photoredox Catalysts Induces Potent Photoreductant Activity**
Chernowsky, Colleen P.,Chmiel, Alyah F.,Wickens, Zachary K.
, p. 21418 - 21425 (2021/08/25)
Herein, we disclose that electrochemical...
456-55-3 Process route
-
-
71-43-2,26181-88-4,54682-86-9,13967-78-7,174973-66-1
benzene

-
-
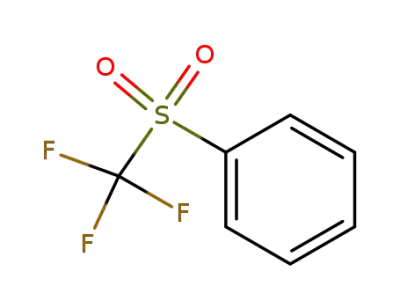
426-58-4
(trifluoromethylsulfonyl)benzene

-
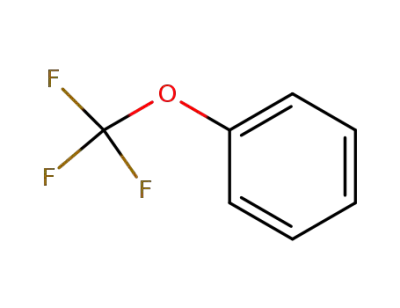
-
456-55-3
1-trifluoromethoxybenzene
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 2 steps
1: boron trifluoride
boron trifluoride;
|
-
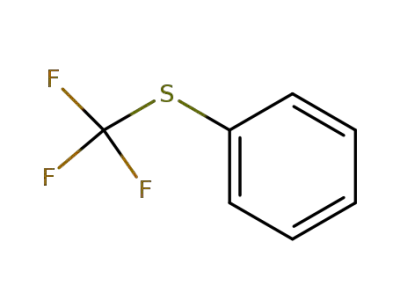
-
456-56-4
phenyl trifluoromethylsulfide

-

-
426-58-4
(trifluoromethylsulfonyl)benzene

-

-
456-55-3
1-trifluoromethoxybenzene
| Conditions | Yield |
|---|---|
|
|
456-55-3 Upstream products
-
34888-05-6
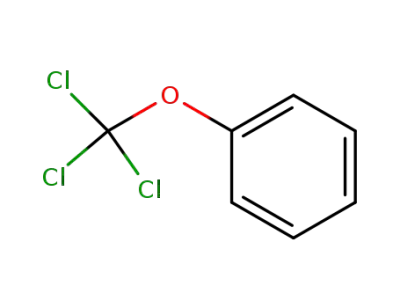
(trichloromethoxy)benzene
-
770-11-6
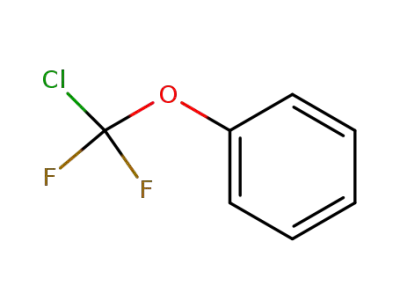
difluorochloromethoxybenzene
-
461-82-5

4-(trifluoromethoxy)aniline
-
461-81-4
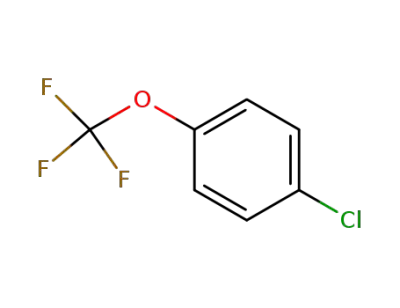
1-chloro-4-(trifluoromethoxy)benzene
456-55-3 Downstream products
-
80258-33-9
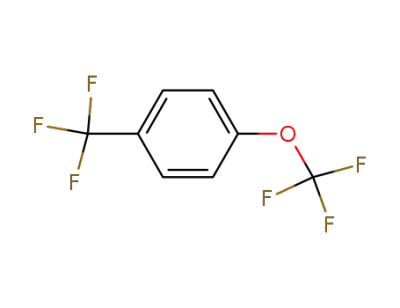
1-(trifluoromethoxy)-4-(trifluoromethyl)benzene
-
1030471-15-8
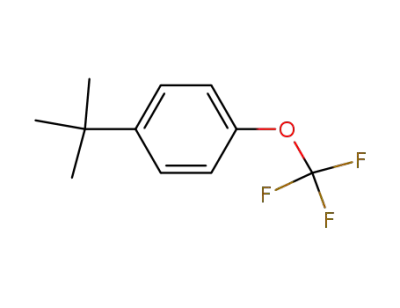
p-tert-butyl-α,α,α-trifluoromethoxybenzene
-
659-28-9
p-trifluoromethoxybenzaldehyde
-
450-96-4
o-chloro-α,α,α-trifluoromethoxybenzene
Relevant Products
-
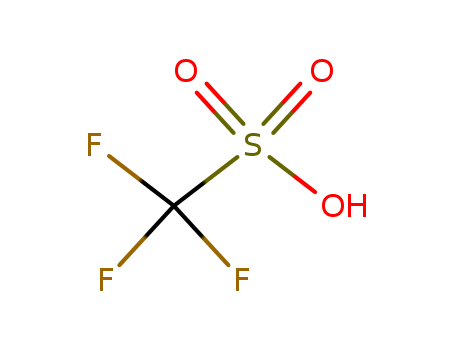
Trifluoromethanesulfonic acid
CAS:1493-13-6
-
1-Ethyl-3-methylimidazolium dicyanamide
CAS:370865-89-7
-
1-Ethyl-3-methylimidazolium tetrafluoroborate
CAS:143314-16-3