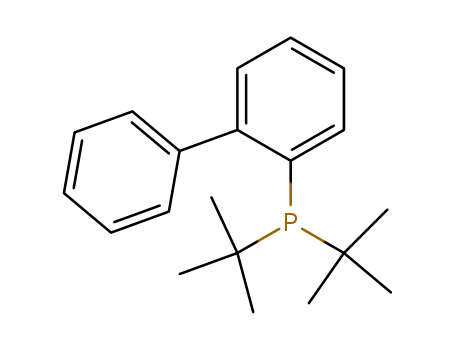
224311-51-7
- Product Name:2-(Di-t-butylphosphino)biphenyl
- Molecular Formula:C20H27P
- Purity:99%
- Molecular Weight:298.408
Product Details;
CasNo: 224311-51-7
Molecular Formula: C20H27P
Appearance: white to light yellow crystal powder
factory and supplier 224311-51-7 2-(Di-t-butylphosphino)biphenyl in stock
- Molecular Formula:C20H27P
- Molecular Weight:298.408
- Appearance/Colour:white to light yellow crystal powder
- Vapor Pressure:2.05E-06mmHg at 25°C
- Melting Point:86-88 °C(lit.)
- Boiling Point:405.5 °C at 760 mmHg
- Flash Point:210.1 °C
- PSA:13.59000
- Density:1 g/cm3
- LogP:6.05780
2-(Di-tert-butylphosphino)biphenyl(Cas 224311-51-7) Usage
|
Reactions |
Ligand used in the palladium-catalyzed synthesis of aromatic amines from aryl chlorides, bromides and triflates. Ligand employed in a very active and general catalyst for Suzuki coupling reactions using aryl chlorides, bromides and triflates. Ligand used in palladium-catalyzed synthesis of oxindoles from α-chloroacetanilides. Effective ligand used in palladium-catalyzed arylation of thiazoles. Used in the formation of 2-benzylindolines via sequential palladium-catalyzed N-arylation/cyclization/C-arylation. Selective in the palladium-catalyzed arylation of silyl enol ethers formed from copper-catalyzed reduction of enones. |
|
General Description |
JohnPhos is a Buchwald′s sterically bulky biaryl phosphine ligand. It is a reactive dialkylbiaryl phosphine ligand which catalyzes the carbon-nitrogen bond-forming reactions. Coordination chemistry of gold catalysts bearing JohnPhos as ligand has been investigated by NMR spectroscopy. |
InChI:InChI=1/C20H27P/c1-19(2,3)21(20(4,5)6)18-15-11-10-14-17(18)16-12-8-7-9-13-16/h7-15H,1-6H3
224311-51-7 Relevant articles
A Lewis Base Nucleofugality Parameter, NFB, and Its Application in an Analysis of MIDA-Boronate Hydrolysis Kinetics
García-Domínguez, Andrés,Gonzalez, Jorge A.,Leach, Andrew G.,Lloyd-Jones, Guy C.,Nichol, Gary S.,Taylor, Nicholas P.
supporting information, (2022/01/04)
The kinetics of quinuclidine displacemen...
Synthesis of di-tert-butylphenol [...] biphenyl compounds (by machine translation)
-
Paragraph 0012, (2019/03/06)
The invention discloses a method for syn...
A preparation method of compound phosphine benzene apperception (by machine translation)
-
Paragraph 0062; 0063; 0064; 0065; 0066; 0067; 0068; 0069, (2016/11/14)
The invention relates to a method for pr...
Palladium-catalyzed P(O)R2 directed C-H arylation to synthesize electron-rich polyaromatic monophosphorus ligands
Hu, Rong-Bin,Zhang, Heng,Zhang, Xiao-Yu,Yang, Shang-Dong
, p. 2193 - 2195 (2014/02/14)
Palladium-catalyzed arylation of (diisop...
224311-51-7 Process route
-
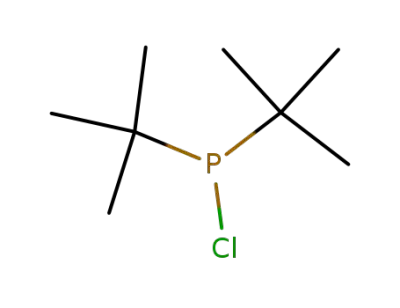
-
13716-10-4
di(tert-butyl)chlorophosphine

-
-
2052-07-5
2-Bromobiphenyl

-
-
224311-51-7
johnphos
| Conditions | Yield |
|---|---|
|
2-Bromobiphenyl;
With
magnesium;
In
tetrahydrofuran;
for 2h;
Inert atmosphere;
Reflux;
di(tert-butyl)chlorophosphine;
With
tetrakis(triphenylphosphine) palladium(0);
In
tetrahydrofuran;
at 20 ℃;
for 2h;
Reagent/catalyst;
Reflux;
Inert atmosphere;
|
95.7% |
|
2-Bromobiphenyl;
With
iodine; magnesium;
In
tetrahydrofuran;
for 2h;
Heating;
di(tert-butyl)chlorophosphine;
With
copper(l) chloride;
In
tetrahydrofuran;
for 8h;
Heating;
|
67% |
|
With
iodine; magnesium; copper(l) chloride;
Yield given;
Multistep reaction;
1.) THF, reflux, 2 h, 2.) THF, reflux, 8 h;
|
-

-
13716-10-4
di(tert-butyl)chlorophosphine

-
-
82214-69-5
2-biphenylmagnesium bromide

-

-
224311-51-7
johnphos
| Conditions | Yield |
|---|---|
|
copper(I) bromide;
In
tetrahydrofuran;
at 30 - 35 ℃;
for 4h;
Heating / reflux;
|
87.5% |
224311-51-7 Upstream products
-
13716-10-4

di(tert-butyl)chlorophosphine
-
2052-07-5
2-Bromobiphenyl
-
82214-69-5

2-biphenylmagnesium bromide
-
1336-21-6
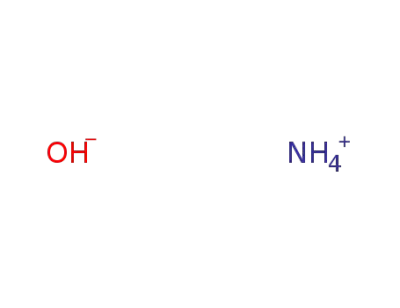
ammonium hydroxide
224311-51-7 Downstream products
-
622391-71-3
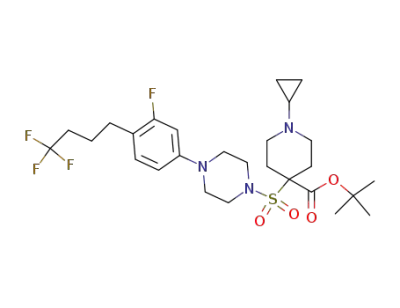
1-cyclopropyl-4-{4-[3-fluoro-4-(4,4,4-trifluoro-butyl)-phenyl]-piperazine-1-sulfonyl}-piperidine-4-carboxylic acid tert-butyl ester
-
395101-91-4
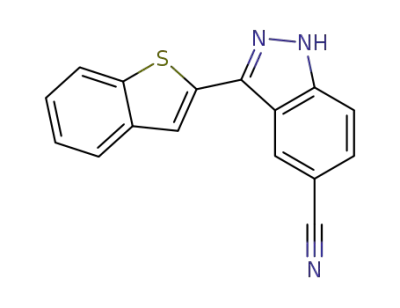
3-benzo[b]thiophen-2-yl-1H-indazole-5-carbonitrile
-
699014-98-7
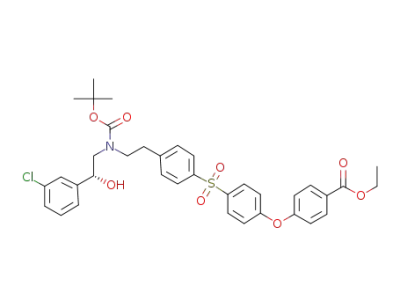
ethyl 4-[4-[[4-[2-[(tert-butoxycarbonyl)[(2R)-2-(3-chlorophenyl)-2-hydroxyethyl]-amino]ethyl]phenyl]sulfonyl]phenoxy]benzoate
-
548465-92-5
5-[3-(3-trifluoromethylphenoxy)phenoxy]isophthalonitrile
Relevant Products
-
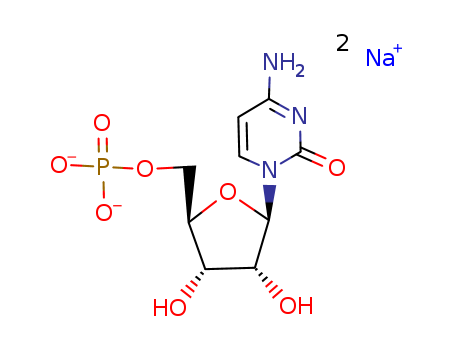
Cytidine 5'-monophosphate disodium salt
CAS:6757-06-8
-
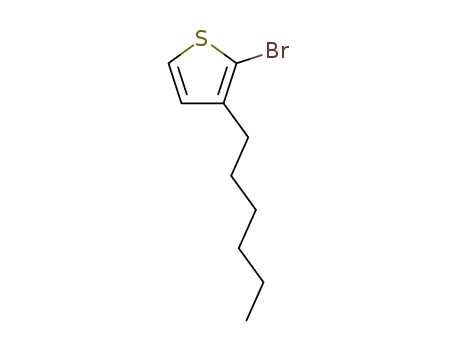
2-Bromo-3-hexylthiophene
CAS:69249-61-2
-
1-Bromopyrene
CAS:1714-29-0