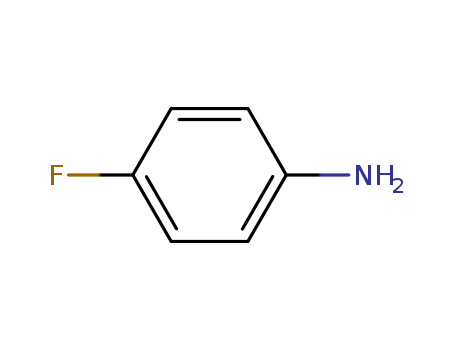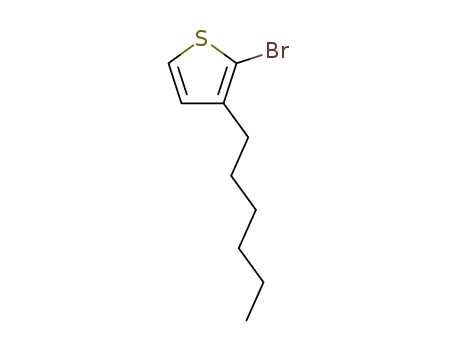
69249-61-2
- Product Name:2-Bromo-3-hexylthiophene
- Molecular Formula:C10H15BrS
- Purity:99%
- Molecular Weight:247.199
Product Details;
CasNo: 69249-61-2
Molecular Formula: C10H15BrS
factory and supplier 69249-61-2 2-Bromo-3-hexylthiophene in stock
- Molecular Formula:C10H15BrS
- Molecular Weight:247.199
- Vapor Pressure:0.01mmHg at 25°C
- Refractive Index:n20/D 1.529
- Boiling Point:272.653 °C at 760 mmHg
- Flash Point:118.697 °C
- PSA:28.24000
- Density:1.275 g/cm3
- LogP:4.63340
2-bromo-3-hexylthiophene(Cas 69249-61-2) Usage
|
General Description |
2-Bromo-3-hexylthiophene is a monomeric precursor that forms bromo terminate polymers. It is synthesized by the bromination of hexylthiophene. |
InChI:InChI=1/C10H15BrS/c1-2-3-4-5-6-9-7-8-12-10(9)11/h7-8H,2-6H2,1H3
69249-61-2 Relevant articles
Push-pull thiophene-based small molecules with donor and acceptor units of varying strength for photovoltaic application: Beyond P3HT and PCBM
Boschi, Alex,Candini, Andrea,Di Maria, Francesca,Gazzano, Massimo,Lanzi, Massimiliano,Marinelli, Martina,Monti, Filippo,Pierini, Filippo,Salatelli, Elisabetta,Zanelli, Alberto,Zangoli, Mattia
supporting information, p. 11216 - 11228 (2021/09/15)
Here is reported an expedient synthesis ...
Redox-Divergent Construction of (Dihydro)thiophenes with DMSO
Chen, Qing-An,He, Gu-Cheng,Hu, Yan-Cheng,Ji, Ding-Wei,Liu, Heng,Zhang, Xiang-Xin,Zhao, Chao-Yang
supporting information, p. 24284 - 24291 (2021/10/08)
Thiophene-based rings are one of the mos...
Planar and twisted molecular structure leads to the high brightness of semiconducting polymer nanoparticles for NIR-IIa fluorescence imaging
Liu, Shunjie,Ou, Hanlin,Li, Yuanyuan,Zhang, Haoke,Liu, Junkai,Lu, Xuefeng,Kwok, Ryan T.K.,Lam, Jacky W.Y.,Ding, Dan,Tang, Ben Zhong
supporting information, p. 15146 - 15156 (2020/10/13)
Semiconducting polymer nanoparticles (SP...
Aggregation induced emission-emissive stannoles in the solid state
Lork, Enno,Ramirez Y Medina, Isabel-Maria,Rohdenburg, Markus,Staubitz, Anne
supporting information, p. 9775 - 9778 (2020/09/07)
The optoelectronic and structural proper...
69249-61-2 Process route
-

-
1693-86-3
3-hexylthiophene

-
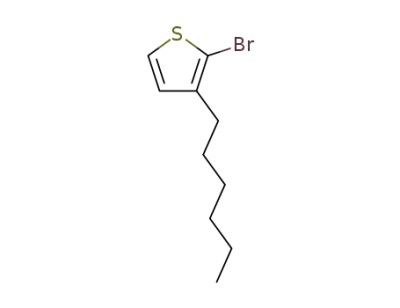
-
69249-61-2,125321-66-6
3-hexyl-2-bromothiophene
| Conditions | Yield |
|---|---|
|
With
N-Bromosuccinimide;
In
acetic acid;
at 20 ℃;
for 24h;
|
99% |
|
With
N-Bromosuccinimide;
In
N,N-dimethyl-formamide;
at 0 - 20 ℃;
Darkness;
|
99% |
|
With
N-Bromosuccinimide; acetic acid;
In
chloroform;
at 20 ℃;
for 3h;
Cooling with ice;
|
99% |
|
With
N-Bromosuccinimide; acetic acid;
In
chloroform;
at 20 ℃;
|
98% |
|
With
N-Bromosuccinimide;
|
98% |
|
With
N-Bromosuccinimide;
In
dichloromethane; acetic acid;
|
98% |
|
With
N-Bromosuccinimide;
In
chloroform; acetic acid;
|
97% |
|
With
N-Bromosuccinimide;
In
N,N-dimethyl-formamide;
at -20 - 20 ℃;
for 1h;
|
97% |
|
With
N-Bromosuccinimide;
In
acetic acid;
|
96% |
|
With
N-Bromosuccinimide;
In
acetic acid;
Inert atmosphere;
|
96% |
|
With
N-Bromosuccinimide;
In
chloroform; acetic acid;
at 20 ℃;
|
96% |
|
With
N-Bromosuccinimide;
In
tetrahydrofuran;
at 0 ℃;
for 1h;
|
95% |
|
With
N-Bromosuccinimide;
In
tetrahydrofuran;
for 1h;
cooling;
|
95% |
|
With
N-Bromosuccinimide;
In
N,N-dimethyl-formamide;
for 3h;
|
95% |
|
With
N-Bromosuccinimide; acetic acid;
In
chloroform;
at 0 - 20 ℃;
for 0.5h;
|
95% |
|
With
N-Bromosuccinimide; acetic acid;
at 20 ℃;
for 24h;
Inert atmosphere;
|
95% |
|
With
N-Bromosuccinimide;
In
tetrahydrofuran;
at 0 ℃;
for 1h;
Inert atmosphere;
|
92% |
|
With
N-Bromosuccinimide;
In
N,N-dimethyl-formamide;
at -10 - 20 ℃;
for 13h;
Inert atmosphere;
Schlenk technique;
Large scale;
|
91% |
|
With
N-Bromosuccinimide; acetic acid;
|
91% |
|
With
N-Bromosuccinimide;
In
acetic acid;
at 0 ℃;
for 2h;
|
89% |
|
With
N-Bromosuccinimide;
In
chloroform; acetic acid;
for 0.5h;
|
88% |
|
With
N-Bromosuccinimide;
In
acetic acid;
for 0.5h;
|
86% |
|
With
N-Bromosuccinimide;
In
tetrahydrofuran;
at 0 ℃;
for 2h;
|
86% |
|
With
N-Bromosuccinimide;
In
tetrahydrofuran;
at 0 ℃;
for 12h;
|
86% |
|
With
N-Bromosuccinimide; acetic acid;
In
chloroform;
at 0 - 22 ℃;
for 3.16667h;
|
84% |
|
With
trimethylsilyl bromide; bis-[(trifluoroacetoxy)iodo]benzene;
In
dichloromethane;
at 20 ℃;
|
81% |
|
With
N-Bromosuccinimide;
In
chloroform; acetic acid;
at 20 - 50 ℃;
for 0.5h;
|
79% |
|
With
N-Bromosuccinimide;
In
N,N-dimethyl-formamide;
at 0 ℃;
|
78% |
|
With
N-Bromosuccinimide;
In
acetic acid;
at 15 ℃;
for 2.5h;
|
78% |
|
With
N-Bromosuccinimide;
In
acetic acid;
at 22 ℃;
for 2.5h;
|
77% |
|
With
N-Bromosuccinimide;
In
N,N-dimethyl-formamide;
at 0 - 20 ℃;
for 25h;
Darkness;
|
77% |
|
With
N-Bromosuccinimide;
In
tetrahydrofuran;
at 0 ℃;
for 2h;
Inert atmosphere;
|
70% |
|
With
N-Bromosuccinimide; acetic acid;
In
chloroform;
at 0 ℃;
for 1h;
|
67% |
|
With
N-Bromosuccinimide;
In
N,N-dimethyl-formamide;
at -20 - 20 ℃;
for 5.5h;
|
52% |
|
With
bromine;
In
acetic acid;
at 0 ℃;
for 0.5h;
|
49% |
|
With
N-Bromosuccinimide;
In
N,N-dimethyl-formamide;
at 20 ℃;
Inert atmosphere;
|
47% |
|
With
N-Bromosuccinimide;
In
chloroform; acetic acid;
|
|
|
With
N-Bromosuccinimide;
In
N,N-dimethyl-formamide;
|
|
|
Multi-step reaction with 2 steps
1.1: 95 percent / tetrabutylammonium bromide; methanol; Br2 / CH2Cl2 / 20 °C
2.1: ZnBr2; tetrabutylammonium tetrafluoroborate / NiBr2 2,2'-bipyridine / dimethylformamide / -10.16 °C / Electrolysis
2.2: dimethylformamide / Acid hydrolysis
With
methanol; tetrabutylammomium bromide; tetrabutylammonium tetrafluoroborate; bromine; zinc dibromide;
(2,2'-bipyridine)nickel(II) dibromide;
In
dichloromethane; N,N-dimethyl-formamide;
|
|
|
With
N-Bromosuccinimide;
In
tetrahydrofuran;
at 20 ℃;
for 15h;
|
|
|
With
N-Bromosuccinimide;
In
dichloromethane;
|
|
|
With
N-Bromosuccinimide;
In
N,N-dimethyl-formamide;
at 20 ℃;
for 4h;
Inert atmosphere;
|
|
|
With
N-Bromosuccinimide; acetic acid;
In
dichloromethane;
at -20 ℃;
Inert atmosphere;
|
|
|
With
N-Bromosuccinimide;
In
tetrahydrofuran;
|
|
|
With
N-Bromosuccinimide;
In
tetrahydrofuran;
at 25 ℃;
for 15h;
|
|
|
With
N-Bromosuccinimide; acetic acid;
at 20 ℃;
for 0.5h;
Inert atmosphere;
Darkness;
|
|
|
With
N-Bromosuccinimide;
In
tetrahydrofuran;
at 0 ℃;
for 1h;
Cooling with ice;
|
|
|
With
N-Bromosuccinimide;
In
dichloromethane;
Darkness;
Inert atmosphere;
|
|
|
With
N-Bromosuccinimide;
In
dichloromethane; acetic acid;
for 6h;
|
|
|
With
N-Bromosuccinimide;
In
tetrahydrofuran;
at 0 ℃;
for 1h;
|
|
|
With
N-Bromosuccinimide;
In
tetrahydrofuran;
at 0 - 20 ℃;
for 2h;
Inert atmosphere;
|
|
|
With
N-Bromosuccinimide;
In
chloroform;
at -90 - 20 ℃;
for 15h;
|
|
|
With
N-Bromosuccinimide;
at 0 ℃;
|
|
|
With
N-Bromosuccinimide;
|
|
|
With
hydrogen bromide; acetic acid; dimethyl sulfoxide;
at 60 ℃;
for 6h;
Sealed tube;
|
5.1 mg |
-
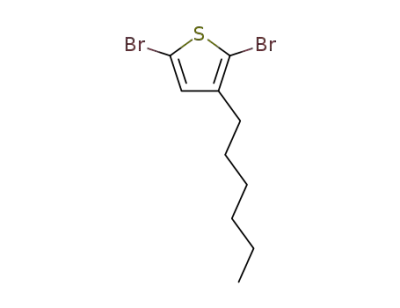
-
116971-11-0
2,5-dibromo-3-hexylthiophene

-

-
69249-61-2,125321-66-6
3-hexyl-2-bromothiophene
| Conditions | Yield |
|---|---|
|
2,5-dibromo-3-hexylthiophene;
With
tetrabutylammonium tetrafluoroborate; zinc dibromide;
(2,2'-bipyridine)nickel(II) dibromide;
In
N,N-dimethyl-formamide;
at -10.16 ℃;
Electrolysis;
In
N,N-dimethyl-formamide;
Further stages.;
Acid hydrolysis;
|
69249-61-2 Upstream products
-
1693-86-3

3-hexylthiophene
-
116971-11-0

2,5-dibromo-3-hexylthiophene
-
68-12-2
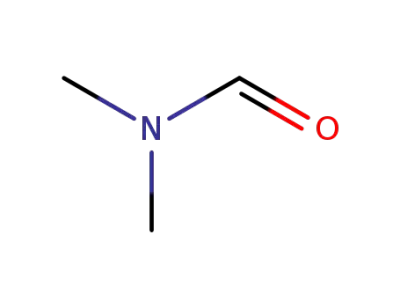
N,N-dimethyl-formamide
-
3761-92-0
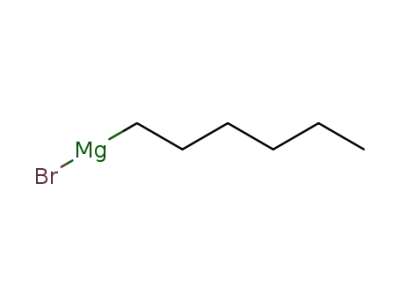
n-hexylmagnesium bromide
69249-61-2 Downstream products
-
132400-58-9
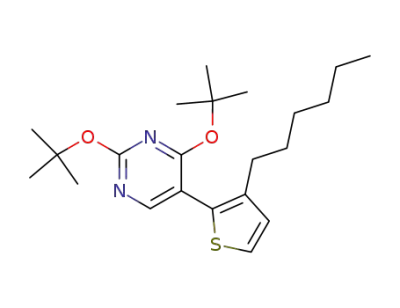
2,4-Di-tert-butoxy-5-(3-hexyl-thiophen-2-yl)-pyrimidine
-
160096-76-4
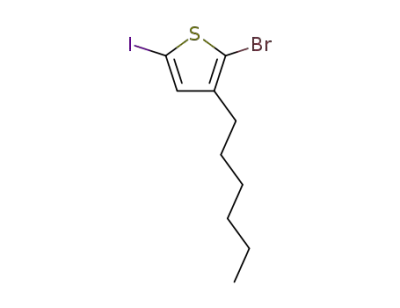
2-bromo-5-iodo-3-hexylthiophene
-
1693-86-3

3-hexylthiophene
-
125607-30-9

3,3'-dihexyl-2,2'-bithiophene
Relevant Products
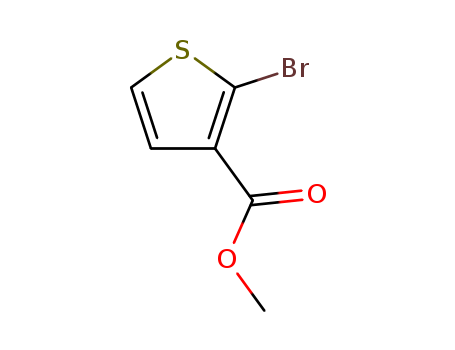
-
Methyl 2-bromothiophene-3-carboxylate
CAS:76360-43-5
-
4-Fluoroaniline
CAS:371-40-4
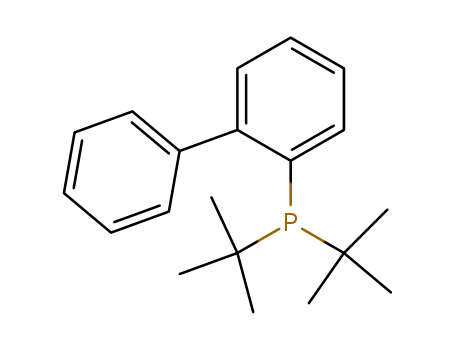
-
2-(Di-t-butylphosphino)biphenyl
CAS:224311-51-7