1714-29-0
- Product Name:1-Bromopyrene
- Molecular Formula:C16H9Br
- Purity:99%
- Molecular Weight:281.151
Product Details;
CasNo: 1714-29-0
Molecular Formula: C16H9Br
Appearance: light yellow solid
factory and supplier 1714-29-0 1-Bromopyrene in stock
- Molecular Formula:C16H9Br
- Molecular Weight:281.151
- Appearance/Colour:light yellow solid
- Vapor Pressure:5.91E-07mmHg at 25°C
- Melting Point:102-105 °C
- Refractive Index:1.858
- Boiling Point:422.5 °C at 760 mmHg
- Flash Point:209.4 °C
- PSA:0.00000
- Density:1.578 g/cm3
- LogP:5.34650
1-Bromopyrene(Cas 1714-29-0) Usage
|
Preparation |
1-Bromopyrene is an important intermediate in the OLED material industry. The synthesis method is as follows: pyrene is dissolved in an organic solvent dichloromethane, and dibromohydantoin is added for reaction, filtered, and the obtained solid is recrystallized to obtain 1-bromopyrene. |
|
Synthesis Reference(s) |
Synthetic Communications, 18, p. 2207, 1988 DOI: 10.1080/00397918808082362Synthesis of 1-Bromopyrene and 1-Pyrenecarbaldehyde |
|
General Description |
1-Bromopyrene, a polycyclic aromatic hydrocarbon (PAH), is a mono bromo substituted pyrene derivative. Its synthesis has been reported. Its gas phase UV-absorption spectra in the UV wavelength range at elevated temperature has been studied. Electron affinitiy (EA) of 1-bromopyrene has been investigated using electron attachment desorption chemical ionization mass spectrometry (DCI-MS) and triple quadrupole tandem mass spectrometry. It participates in the synthesis of novel ruthenium (II) bipyridine or terpyridine complexes bearing pyrene moiety. The reaction of 1-bromopyrene cation radical with water in acetonitrile has been analyzed using the electron transfer stopped-flow (ETSF) method. |
InChI:InChI=1/C16H9Br/c17-14-9-12-5-1-3-10-7-8-11-4-2-6-13(14)16(11)15(10)12/h1-9H
1714-29-0 Relevant articles
Effects of substituents on absorption and fluorescence properties of trimethylsilylethynyl- and tert-butylethynyl-pyrenes
Furuyama, Taniyuki,Maeda, Hajime,Segi, Masahito,Ueno, Ryota
, (2020)
Effects of substituents introduced at 1-...
Pyrene-Oxadiazoles for Organic Light-Emitting Diodes: Triplet to Singlet Energy Transfer and Role of Hole-Injection/Hole-Blocking Materials
Chidirala, Swetha,Ulla, Hidayath,Valaboju, Anusha,Kiran, M. Raveendra,Mohanty, Maneesha Esther,Satyanarayan,Umesh,Bhanuprakash, Kotamarthi,Rao, Vaidya Jayathirtha
, p. 603 - 614 (2016)
Three pyrene-oxadiazole derivatives were...
Novel ethynyl-pyrene substituted phenothiazine based metal free organic dyes in DSSC with 12% conversion efficiency
Nagarajan, Bhanumathi,Kushwaha, Suman,Elumalai, Ramachandran,Mandal, Sudip,Ramanujam, Kothandaraman,Raghavachari, Dhamodharan
, p. 10289 - 10300 (2017)
Six new dyes based on phenothiazine conj...
A mechanistically-distinct approach to fluorescence visualization of singlet oxygen
Yang,Finney
, p. 11449 - 11452 (2017)
We describe fluorescence detection of 1O...
3-pyrenylacrylates: Synthetic, photophysical, theoretical and electrochemical investigations
Reimann, Sebastian,Sharif, Muhammad,Wittler, Kai,Knoepke, Leif R.,Surkus, Annette-E.,Roth, Christian,Ludwig, Ralf,Langer, Peter
, p. 367 - 377 (2013)
The Mizoroki-Heck coupling of 1-bromopyr...
A pyrene-based dual chemosensor for colorimetric detection of Cu2+ and fluorescent detection of Fe3+
Guo, Yuxin,Wang, Lei,Zhuo, Jiezhen,Xu, Bo,Li, Xue,Zhang, Jianyu,Zhang, Zhiqiang,Chi, Haijun,Dong, Yan,Lu, Gonghao
, p. 3951 - 3956 (2017)
A pyrene based chemosensor was designed ...
Pyrene terminal functionalized perylene diimide as non-fullerene acceptors for bulk heterojunction solar cells
Liu, Xin,Luo, Guoping,Cai, Xinyi,Wu, Hongbin,Su, Shi-Jian,Cao, Yong
, p. 83155 - 83163 (2015)
Two perylene diimide (PDI) based small m...
Pseudo-dumbbell-type molecular beacon probes bearing modified deoxyuridine derivatives and a silylated pyrene as a fluorophore
Chowdhury, Jakir Ahmed,Moriguchi, Tomohisa,Shinozuka, Kazuo
, p. 496 - 502 (2015)
We have recently reported a novel pseudo...
The synthesis of polyarene-modified 5-phenyl-2,2'-bipyridines via the methodology and aza-Diels-Alder reaction
Kovalev, Igor S.,Kopchuk, Dmitry S.,Khasanov, Albert F.,Zyryanov, Grigory V.,Rusinov, Vladimir L.,Chupakhin, Oleg N.
, p. 117 - 118 (2014)
Nucleophilic substitution of hydrogen ()...
ON–OFF Fluorescent Imidazole Derivative for Sensitive and Selective Detection of Copper(II) Ions
Appalanaidu, E.,Baggi, T. R.,Harsha, K. G.,Rao, B. A.,Rao, V. J.
, p. 158 - 168 (2020)
A novel multichromophoric hybrid compoun...
Synthesis and optoelectronic properties of novel organosoluble polynorbornenes containing asymmetric pyrenyl and electroactive substituents via ring-opening metathesis polymerization
Lian, Wei-Ren,Ho, Crystal,Huang, Ying-Chi,Liao, Yi-An,Wang, Kun-Li,Liaw, Der-Jang,Lee, Kueir-Rarn,Lai, Juin-Yih
, p. 5350 - 5357 (2011)
Novel polynorbornenes, poly(NBPYTPA), an...
Self-Assembly of “Chalcone” Type Push-Pull Dye Molecules into Organic Single Crystalline Microribbons and Rigid Microrods for Vis/NIR Range Photonic Cavity Applications
Vattikunta, Radhika,Venkatakrishnarao, Dasari,Mohiddon, Mahamad Ahamad,Chandrasekar, Rajadurai
, p. 3435 - 3441 (2016)
A novel supramolecular fluorescent donor...
Bis-selenonium Cations as Bidentate Chalcogen Bond Donors in Catalysis
He, Xinxin,Wang, Xinyan,Tse, Ying-Lung Steve,Ke, Zhihai,Yeung, Ying-Yeung
, p. 12632 - 12642 (2021/10/21)
Lewis acids are frequently employed in c...
Method for preparing hydroxypyrene compound with high purity
-
Paragraph 0036-0038, (2021/03/16)
The present invention relates to a metho...
Naphthoselenodiazole delayed-fluorescence material and preparation method thereof
-
Paragraph 0016-0018, (2021/07/28)
The invention belongs to the technical f...
Thermally activated delayed fluorescent material and preparation method thereof
-
Paragraph 0017-0019, (2021/07/31)
The invention relates to the technical f...
1714-29-0 Process route
-
-
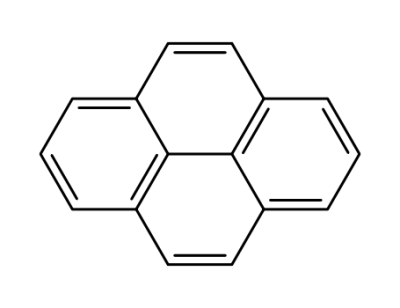
129-00-0
pyrene

-
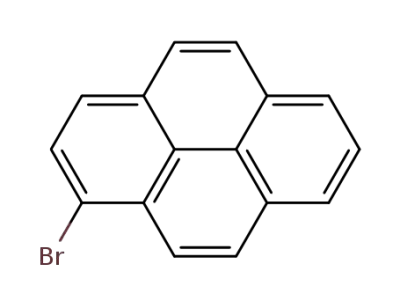
-
1714-29-0
1-bromopyrene
| Conditions | Yield |
|---|---|
|
With
N-Bromosuccinimide;
In
dichloromethane;
for 2h;
|
100% |
|
With
hydrogen bromide; dihydrogen peroxide;
In
methanol; diethyl ether; water;
at 15 - 20 ℃;
for 12.25h;
|
96% |
|
With
N-Bromosuccinimide; dibenzoyl peroxide;
In
N,N-dimethyl-formamide;
at 20 ℃;
|
96% |
|
With
hydrogen bromide; dihydrogen peroxide;
In
methanol; diethyl ether;
at 20 ℃;
for 12h;
|
95% |
|
With
hydrogen bromide; dihydrogen peroxide;
In
methanol; diethyl ether; water;
at 10 - 20 ℃;
for 14.25h;
Inert atmosphere;
Pressure tube;
|
95% |
|
With
hydrogen bromide; dihydrogen peroxide;
In
methanol; diethyl ether; water;
at 10 - 20 ℃;
for 14h;
Inert atmosphere;
|
95% |
|
With
N-Bromosuccinimide;
In
dichloromethane;
at 20 ℃;
for 6h;
|
95% |
|
With
N-Bromosuccinimide;
In
dichloromethane;
for 12h;
Darkness;
|
95% |
|
With
N-Bromosuccinimide; C28H28Se2(2+)*2BF4(1-);
In
dichloromethane;
at -40 ℃;
for 72h;
Darkness;
|
95% |
|
With
N-Bromosuccinimide; methyl bis[4-(trifluoromethyl)phenyl]selenonium tetrafluoroborate;
In
dichloromethane;
at -40 ℃;
for 72h;
Inert atmosphere;
Darkness;
|
91% |
|
With
hydrogen bromide; dihydrogen peroxide;
In
methanol; diethyl ether; water;
at 12 - 30 ℃;
for 12.3333h;
|
90% |
|
With
N-Bromosuccinimide;
In
dichloromethane;
at 20 ℃;
for 2h;
|
90% |
|
With
hydrogen bromide; dihydrogen peroxide;
In
methanol; diethyl ether; water;
at 20 ℃;
for 12.25h;
Inert atmosphere;
Cooling;
|
90% |
|
With
N-Bromosuccinimide;
In
dichloromethane;
at 20 ℃;
for 6h;
regioselective reaction;
|
88% |
|
With
bromine;
In
tetrachloromethane;
|
86% |
|
With
hydrogen bromide; dihydrogen peroxide;
In
methanol; diethyl ether; water;
for 15.25h;
|
86% |
|
With
hydrogen bromide; dihydrogen peroxide;
In
methanol;
at 10 ℃;
for 9h;
|
86% |
|
With
N-Bromosuccinimide;
In
N,N-dimethyl-formamide;
at 20 ℃;
for 24h;
|
85% |
|
With
N-Bromosuccinimide;
In
N,N-dimethyl-formamide;
at 0 - 20 ℃;
for 24h;
|
85.4% |
|
With
hydrogen bromide; dihydrogen peroxide;
In
methanol; diethyl ether;
at 0 ℃;
for 8h;
|
85% |
|
With
hydrogen bromide; dihydrogen peroxide;
In
methanol; diethyl ether;
at 0 ℃;
for 8h;
|
85% |
|
With
hydrogen bromide; dihydrogen peroxide;
In
methanol; diethyl ether;
at 0 ℃;
for 8h;
|
85% |
|
With
hydrogen bromide; dihydrogen peroxide;
In
methanol; diethyl ether;
at 0 ℃;
for 8h;
|
85% |
|
With
hydrogen bromide;
In
methanol; diethyl ether; dihydrogen peroxide;
at 0 ℃;
for 8h;
|
85% |
|
With
hydrogen bromide; dihydrogen peroxide;
In
methanol; diethyl ether;
at 0 ℃;
for 8h;
|
85% |
|
With
hydrogen bromide; dihydrogen peroxide;
In
methanol; diethyl ether;
at 0 ℃;
for 8h;
|
85% |
|
With
hydrogen bromide; dihydrogen peroxide;
In
methanol; diethyl ether;
at 0 ℃;
for 8h;
|
85% |
|
With
hydrogen bromide; dihydrogen peroxide;
In
methanol; diethyl ether;
at 0 ℃;
for 8h;
|
85% |
|
pyrene;
With
hydrogen bromide;
In
methanol; diethyl ether;
at 10 - 15 ℃;
With
dihydrogen peroxide;
In
methanol; diethyl ether;
at 20 ℃;
for 24h;
|
84.4% |
|
With
hydrogen bromide; dihydrogen peroxide;
In
tetrahydrofuran; methanol; water;
at 20 ℃;
for 25h;
Cooling with ice;
|
84.7% |
|
With
tetrachloromethane; bromine;
at 20 ℃;
|
82% |
|
With
bromine;
In
chloroform;
at 80 ℃;
for 24h;
regioselective reaction;
Inert atmosphere;
|
81% |
|
With
benzyltrimethylammonium tribromide; calcium carbonate;
In
methanol; dichloromethane;
for 4h;
Ambient temperature;
|
80.3% |
|
With
N-Bromosuccinimide;
In
N,N-dimethyl-formamide;
at 20 ℃;
|
78% |
|
With
mono(N,N,N-trimethylbenzenaminium) tribromide;
In
dichloromethane;
at 20 ℃;
for 5h;
Inert atmosphere;
|
78% |
|
With
hydrogen bromide; dihydrogen peroxide;
In
methanol; diethyl ether; water;
at 15 - 20 ℃;
for 16h;
|
77% |
|
With
N-Bromosuccinimide;
In
chloroform;
at 65 ℃;
for 10h;
Temperature;
Inert atmosphere;
|
77% |
|
With
bromine;
In
dichloromethane;
at -78 - 20 ℃;
Inert atmosphere;
Schlenk technique;
|
75% |
|
With
N-Bromosuccinimide;
In
chloroform;
at 65 ℃;
for 10h;
Temperature;
Inert atmosphere;
|
75% |
|
With
N-Bromosuccinimide;
In
chloroform;
at 65 ℃;
Inert atmosphere;
Reflux;
|
72% |
|
With
hydrogen bromide; dihydrogen peroxide;
In
methanol; diethyl ether;
at 0 ℃;
for 8h;
|
71% |
|
With
bromine;
In
tetrachloromethane;
at 80 ℃;
for 12h;
|
70% |
|
With
acetic acid; potassium iodide;
In
dichloromethane; water;
at 50 ℃;
for 0.25h;
|
68% |
|
With
acetic acid; mono(N,N,N-trimethylbenzenaminium) tribromide; zinc(II) chloride;
at 20 ℃;
for 12h;
|
67% |
|
With
tetrachloromethane; bromine;
|
|
|
With
chloroform; bromine;
|
|
|
With
water; bromine;
|
|
|
With
tetrachloromethane; N-Bromosuccinimide;
|
|
|
With
N-Bromosuccinimide; benzene;
|
|
|
With
pyridine; bromine;
|
|
|
With
N-Bromosuccinimide;
In
N,N-dimethyl-formamide;
|
|
|
With
N-Bromosuccinimide;
In
N,N-dimethyl-formamide;
at 20 ℃;
for 24h;
|
|
|
With
N-Bromosuccinimide;
In
N,N-dimethyl-formamide;
|
|
|
With
N-Bromosuccinimide;
In
N,N-dimethyl-formamide;
|
|
|
With
bromine;
In
nitrobenzene;
at 20 - 120 ℃;
for 12h;
|
|
|
With
bromine;
In
dichloromethane;
|
|
|
With
hydrogen bromide; dihydrogen peroxide;
In
diethyl ether;
Inert atmosphere;
Schlenk technique;
|
|
|
With
N-Bromosuccinimide;
In
dichloromethane;
at 20 ℃;
for 12h;
|
|
|
With
hydrogen bromide; dihydrogen peroxide;
In
methanol; diethyl ether;
at 0 - 20 ℃;
|
|
|
With
N-Bromosuccinimide;
In
N,N-dimethyl-formamide;
Inert atmosphere;
|
|
|
With
N-Bromosuccinimide;
In
N,N-dimethyl-formamide;
at 0 - 20 ℃;
for 24h;
|
|
|
With
bromine;
In
dichloromethane;
|
|
|
With
N-Bromosuccinimide;
|
|
|
With
N-Bromosuccinimide;
In
chloroform;
at 0 ℃;
|
|
|
With
N-Bromosuccinimide;
In
N,N-dimethyl-formamide;
at 0 - 10 ℃;
for 2h;
Inert atmosphere;
|
|
|
With
pyridine; bromine;
|
|
|
With
chloroform; bromine;
|
|
|
With
water; bromine;
|
|
|
With
hydrogen bromide; dihydrogen peroxide;
In
tetrahydrofuran; methanol;
at 0 - 10 ℃;
|
|
|
With
1,3-dibromo-5,5-dimethylimidazolidine-2,4-dione;
In
1,2-dichloro-ethane;
at 0 - 10 ℃;
for 0.516667h;
|
-
-
C24H29BrO2

-
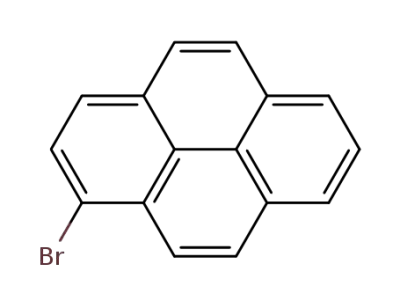
-
1714-29-0
1-bromopyrene
| Conditions | Yield |
|---|---|
|
With
methanesulfonic acid;
In
dichloromethane;
at 0 - 20 ℃;
for 1h;
Inert atmosphere;
|
93.24% |
1714-29-0 Upstream products
-
56-23-5
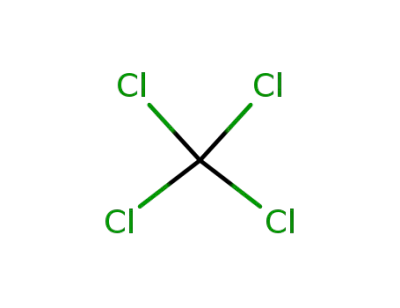
tetrachloromethane
-
128-08-5
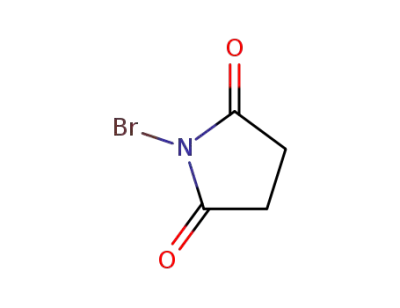
N-Bromosuccinimide
-
129-00-0

pyrene
-
71-43-2
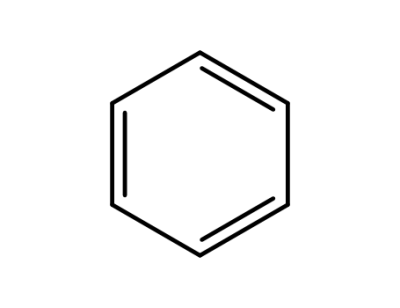
benzene
1714-29-0 Downstream products
-
5101-26-8
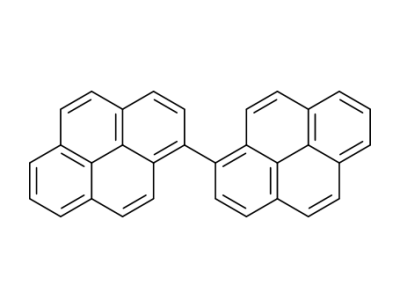
1,1'-dipyrenyl
-
188-90-9
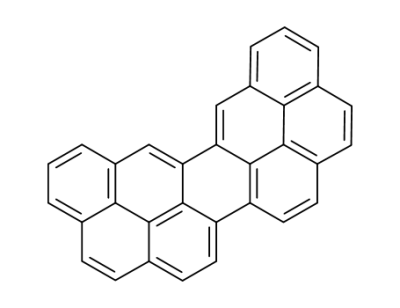
dinaphtho[2,1,8,7-defg:2',1',8',7'-ijkl]pentaphene
-
188-91-0
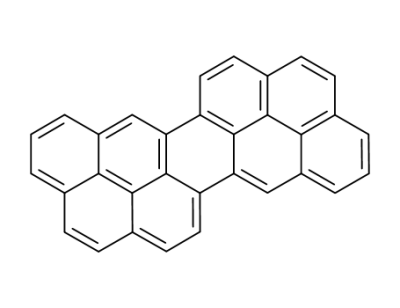
Dinaphtho<2,1,8,7defg;2',1',8',7'opqr>pentacene
-
74391-90-5

1-pyrenyllithium
Relevant Products
-
5-Bromo-2-fluoro-m-xylene
CAS:99725-44-7
-
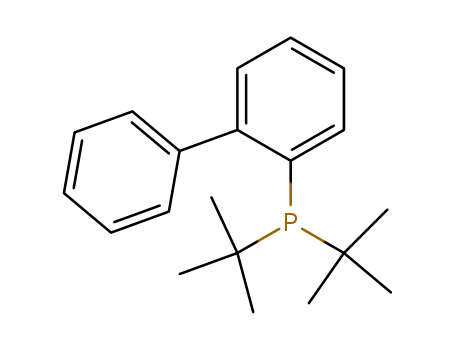
2-(Di-t-butylphosphino)biphenyl
CAS:224311-51-7
-
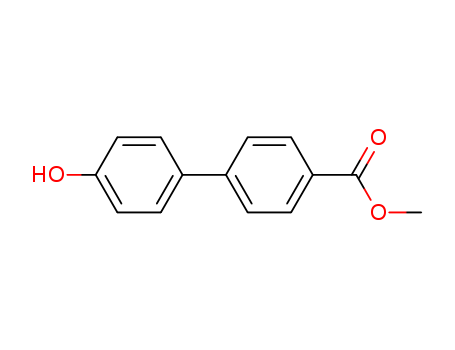
Methyl 4'-hydroxy-4-biphenylcarboxylate
CAS:40501-41-5