76360-43-5
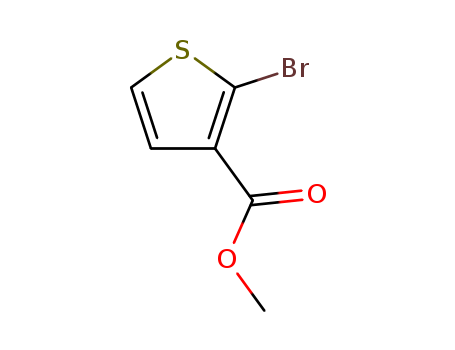
- Product Name:Methyl 2-bromothiophene-3-carboxylate
- Molecular Formula:C6H5BrO2S
- Purity:99%
- Molecular Weight:221.075
Product Details;
CasNo: 76360-43-5
Molecular Formula: C6H5BrO2S
factory and supplier 76360-43-5 Methyl 2-bromothiophene-3-carboxylate in stock
- Molecular Formula:C6H5BrO2S
- Molecular Weight:221.075
- Boiling Point:114-115 °C(Press: 4 Torr)
- PSA:54.54000
- Density:1.662±0.06 g/cm3(Predicted)
- LogP:2.29720
76360-43-5 Relevant articles
PESTICIDALLY ACTIVE HETEROCYCLIC DERIVATIVES WITH SULFUR CONTAINING SUBSTITUENTS
-
Page/Page column 73, (2022/02/05)
Compounds of the formula (I) wherein the...
An attempt to synthesize a terthienyl-based analog of indacenedithiophene (IDT): unexpected synthesis of a naphtho[2,3-b]thiophene derivative
Anghel, C?t?lin C.,Stroia, Ioan,Pop, Alexandra,Bende, Atilla,Grosu, Ion,H?dade, Niculina D.,Roncali, Jean
, p. 9894 - 9900 (2021/03/23)
We report herein our attempt to synthesi...
HISTONE ACETYLTRANSFERASE (HAT) INHIBITOR AND USE THEREOF
-
Paragraph 0722; 0724, (2021/02/25)
The present invention relates to a histo...
p-diaminobenzene derivative as potassium channel regulator, preparation method and medical applications thereof
-
Paragraph 0220-0224, (2019/12/09)
The invention relates to a p-diaminobenz...
76360-43-5 Process route
-
-
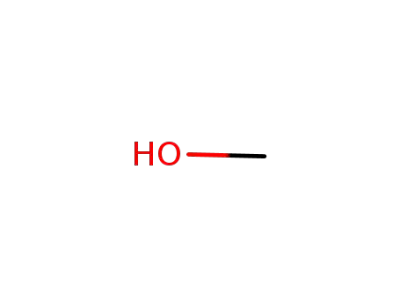
67-56-1
methanol

-
-
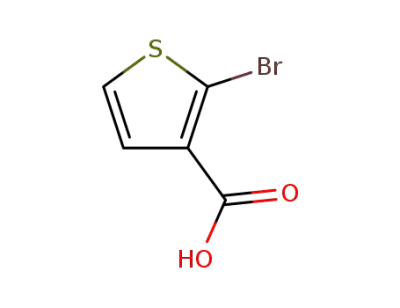
24287-95-4
2-bromothiophene-3-carboxylic acid

-
-
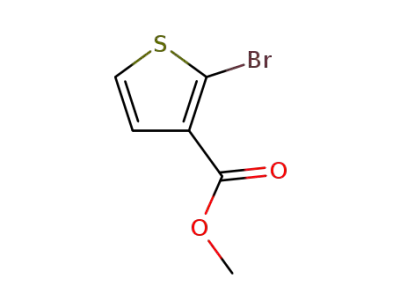
76360-43-5
methyl 2-bromothiophene-3-carboxylate
| Conditions | Yield |
|---|---|
|
With
sulfuric acid;
Reflux;
|
100% |
|
With
sulfuric acid;
for 6h;
Reflux;
|
99% |
|
2-bromothiophene-3-carboxylic acid;
With
oxalyl dichloride;
N,N-dimethyl-formamide;
In
dichloromethane;
at 20 ℃;
methanol;
Heating / reflux;
|
98% |
|
2-bromothiophene-3-carboxylic acid;
With
oxalyl dichloride;
N,N-dimethyl-formamide;
In
dichloromethane;
at 20 ℃;
methanol;
Heating;
|
98% |
|
2-bromothiophene-3-carboxylic acid;
With
oxalyl dichloride;
In
dichloromethane;
at 20 ℃;
Cooling with ice;
methanol;
In
dichloromethane;
for 4h;
Reflux;
|
93.6% |
|
With
sulfuric acid;
|
91% |
|
With
thionyl chloride;
for 72h;
Reflux;
|
80% |
|
With
thionyl chloride;
at 20 ℃;
for 48h;
Inert atmosphere;
|
|
|
With
hydrogenchloride;
at 0 - 60 ℃;
|
|
|
With
hydrogenchloride;
at 0 - 60 ℃;
|
|
|
With
hydrogenchloride;
at 0 - 60 ℃;
|
|
|
With
hydrogenchloride;
at 0 - 60 ℃;
|
|
|
With
hydrogenchloride;
at 0 - 60 ℃;
|
|
|
With
hydrogenchloride;
at 0 - 60 ℃;
|
|
|
With
hydrogenchloride;
at 0 - 60 ℃;
|
|
|
With
hydrogenchloride;
at 0 - 60 ℃;
|
|
|
With
hydrogenchloride;
at 0 - 60 ℃;
|
|
|
With
hydrogenchloride;
at 0 - 60 ℃;
|
|
|
With
thionyl chloride;
at 20 ℃;
for 3.5h;
Reflux;
|
71 g |
|
With
hydrogenchloride;
at 0 - 60 ℃;
|
|
|
With
hydrogenchloride;
at 0 - 60 ℃;
|
|
|
With
hydrogenchloride;
at 60 ℃;
|
|
|
With
hydrogenchloride;
at 0 - 60 ℃;
|
-

-
24287-95-4
2-bromothiophene-3-carboxylic acid

-
-
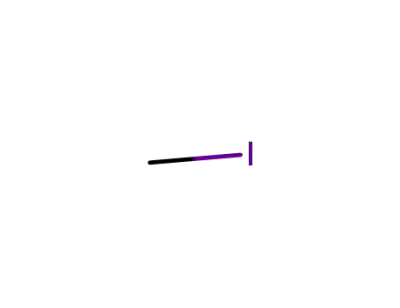
74-88-4
methyl iodide

-

-
76360-43-5
methyl 2-bromothiophene-3-carboxylate
| Conditions | Yield |
|---|---|
|
With
potassium carbonate;
In
N,N-dimethyl-formamide;
at 20 ℃;
for 1h;
|
|
|
With
Cs2CO3;
In
N,N-dimethyl-formamide;
at 20 ℃;
for 5h;
|
|
|
With
Cs2CO3;
In
N,N-dimethyl-formamide;
at 20 ℃;
for 5h;
|
|
|
With
Cs2CO3;
In
N,N-dimethyl-formamide;
at 20 ℃;
for 5h;
|
76360-43-5 Upstream products
-
186581-53-3

diazomethane
-
24287-95-4

2-bromothiophene-3-carboxylic acid
-
89280-91-1
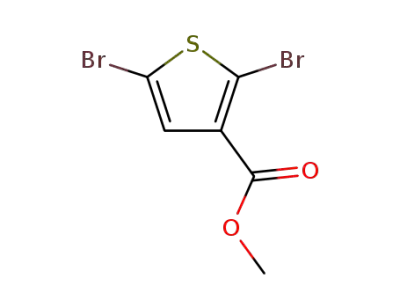
2,5-dibromothiophene-3-carboxylic acid methyl ester
-
67-56-1

methanol
76360-43-5 Downstream products
-
859492-59-4
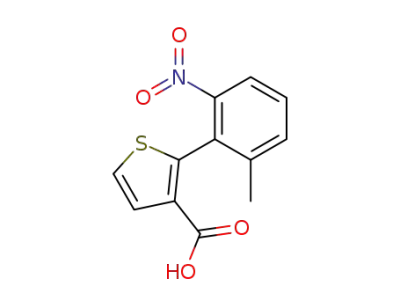
(+/-)-2-(2-methyl-6-nitro-phenyl)-thiophene-3-carboxylic acid
-
1009827-27-3
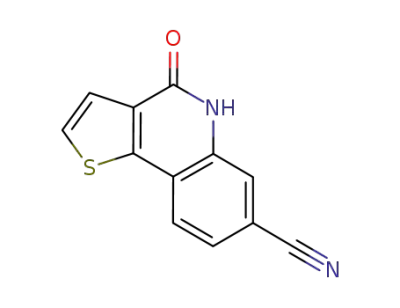
4-oxo-4,5-dihydrothieno[3,2-c]quinoline-7-carbonitrile
-
1395044-64-0
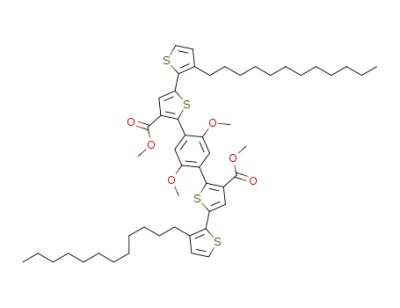
C52H70O6S4
-
1395044-63-9
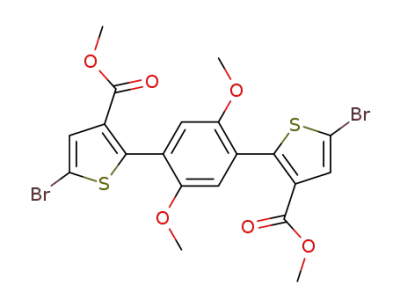
C20H16Br2O6S2
Relevant Products
-
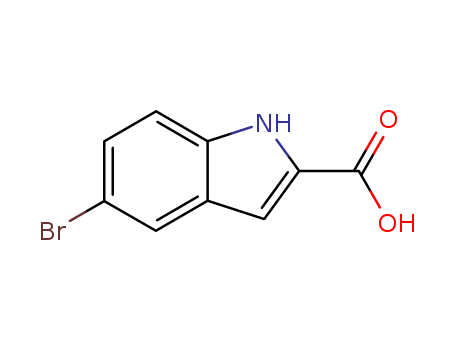
5-Bromoindole-2-carboxylic acid
CAS:7254-19-5
-
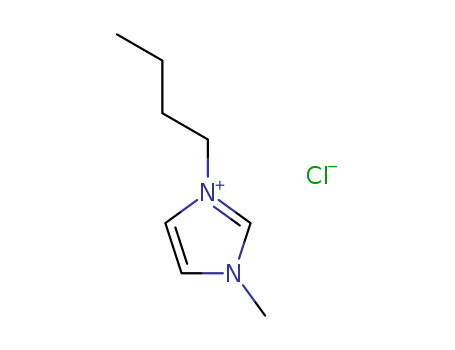
1-Butyl-3-methylimidazolium chloride
CAS:79917-90-1
-
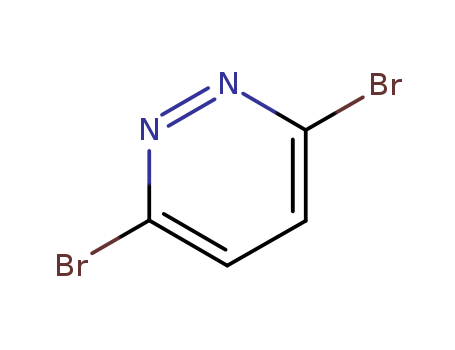
3,6-Dibromopyridazide
CAS:17973-86-3