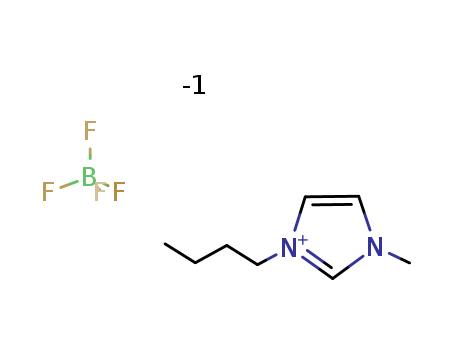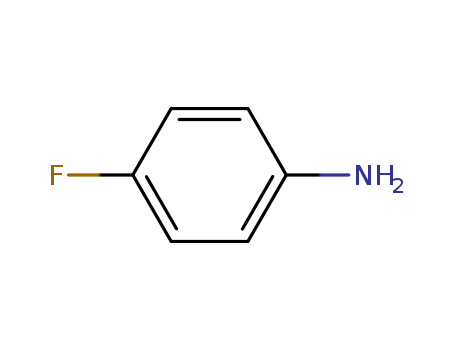
371-40-4
- Product Name:4-Fluoroaniline
- Molecular Formula:C6H6FN
- Purity:99%
- Molecular Weight:111.119
Product Details;
CasNo: 371-40-4
Molecular Formula: C6H6FN
Appearance: light yellow to gold-coloured liquid
factory and supplier 371-40-4 4-Fluoroaniline in stock
- Molecular Formula:C6H6FN
- Molecular Weight:111.119
- Appearance/Colour:light yellow to gold-coloured liquid
- Vapor Pressure:4.04mmHg at 25°C
- Melting Point:-1.9 °C
- Refractive Index:1.5400
- Boiling Point:188.9 °C at 760 mmHg
- PKA:4.65(at 25℃)
- Flash Point:77.9 °C
- PSA:26.02000
- Density:1.158 g/cm3
- LogP:1.98910
4-Fluoroaniline(Cas 371-40-4) Usage
|
Preparation |
The better process in the one-step process is the nitrobenzene method, which is obtained by deoxygenation, hydrogenation and fluorination. With PdCl2-V2O5 as the catalyst and carbon monoxide as the reducing agent, the reaction was carried out at 160°C for 3h, and the yield of 4-fluoroaniline could reach 90%, with another 10% of aniline as a by-product. With PtO2 as the catalyst, BF3-HF as the fluorinating agent and hydrogen as the reducing agent, the reaction was carried out at 42℃ for 12.5h, with 100% conversion and 95% yield. |
|
Synthesis Reference(s) |
Journal of the American Chemical Society, 70, p. 654, 1948 DOI: 10.1021/ja01182a065The Journal of Organic Chemistry, 26, p. 4014, 1961 DOI: 10.1021/jo01068a089 |
|
Hazard |
Toxic material. |
|
Flammability and Explosibility |
Notclassified |
|
Safety Profile |
Poison by ingestion. Mutation data reported. A severe skin and eye irritant. When heated to decomposition it emits very toxic fumes of NOx and F-. |
|
Definition |
ChEBI: 4-fluoroaniline is a primary arylamine that is the derivative of aniline in which the hydrogen at position 4 has been substituted by fluorine. It is used as an intermediate in the manufacture of pharmaceuticals, herbicides and plant growth regulators. It is a primary arylamine and a fluoroaniline. |
|
General Description |
4-fluoroaniline is a light-colored oily liquid. Mixture of three isomers. Insoluble in water and denser than water. Contact may cause irritation to skin, eyes, and mucous membranes. May be toxic by ingestion. Used to make other chemicals. The barriers to inversion and internal rotation of the NH2 group in 4-fluoroaniline has been investigated by far infrared gas spectra. |
InChI:InChI:1S/C6H6FN/c7-5-1-3-6(8)4-2-5/h1-4H,8H2
371-40-4 Relevant articles
-
Fidler et al.
, p. 4014,4016 (1961)
-
Reduction of nitrobenzene derivatives using sodium borohydride and transition metal sulfides
Pi?a, Samuel,Cedillo, Diana M.,Tamez, Carlos,Izquierdo, Nezhueyotl,Parsons, Jason G.,Gutierrez, Jose J.
, p. 5468 - 5470 (2014)
Reported here is the reduction of aromat...
Dihydro-2H-thiopyran-3(4H)-one-1,1-dioxide–a new cyclic ketomethylenic reagent for the Dimroth-type 1,2,3-triazole synthesis
Pokhodylo, Nazariy T.,Tupychak, Mykola A.,Palchykov, Vitalii A.
, p. 1835 - 1844 (2020)
A series of 1,5,6,7-tetrahydrothiopyrano...
Hydrogen Sulfide Donors Activated by Reactive Oxygen Species
Zhao, Yu,Pluth, Michael D.
, p. 14638 - 14642 (2016)
Hydrogen sulfide (H2S) exhibits promisin...
Nickel Boride Catalyzed Reductions of Nitro Compounds and Azides: Nanocellulose-Supported Catalysts in Tandem Reactions
Proietti, Giampiero,Prathap, Kaniraj Jeya,Ye, Xinchen,Olsson, Richard T.,Dinér, Peter
, p. 133 - 146 (2021/11/04)
Nickel boride catalyst prepared in situ ...
Synthesis method of o-bromo-p-fluoroacetyl aniline
-
, (2022/04/03)
The invention relates to a synthesis met...
Aluminum Metal-Organic Framework-Ligated Single-Site Nickel(II)-Hydride for Heterogeneous Chemoselective Catalysis
Antil, Neha,Kumar, Ajay,Akhtar, Naved,Newar, Rajashree,Begum, Wahida,Dwivedi, Ashutosh,Manna, Kuntal
, p. 3943 - 3957 (2021/04/12)
The development of chemoselective and he...
Highly efficient N-doped carbon supported FeSx-Fe2O3 catalyst for hydrogenation of nitroarenes via pyrolysis of sulfurized N,Fe-containing MOFs
Li, Xuewei,She, Wei,Wang, Jing,Li, Weizuo,Li, Guangming
, (2021/05/18)
Integrating MOFs as precursor, especiall...
371-40-4 Process route
-
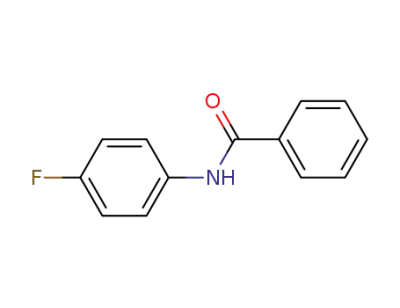
-
366-75-6
N-(4-fluorophenyl)benzamide

-
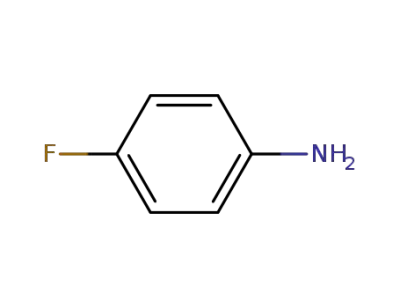
-
371-40-4
4-fluoroaniline

-
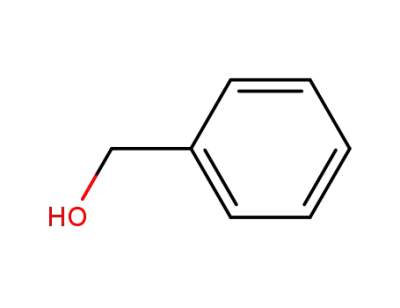
-
100-51-6,185532-71-2
benzyl alcohol
| Conditions | Yield |
|---|---|
|
With
[Ru(η3-C3H5)(Ph2P(CH2)2NH2)2]BF4; potassium tert-butylate; hydrogen;
In
tetrahydrofuran;
at 100 ℃;
for 28h;
under 38002.6 Torr;
Reagent/catalyst;
Time;
|
|
|
With
[fac-8-(2-diphenylphosphinoethyl)aminotrihydroquinoline]RuH(η1-BH4)(CO); hydrogen;
In
isopropyl alcohol;
at 120 ℃;
for 16h;
under 37503.8 Torr;
Concentration;
Autoclave;
|
99 %Chromat. 99 %Chromat. |
|
With
Ag/γ-Al2O3 (2.5 mol%); potassium tert-butylate; hydrogen;
In
1,4-dioxane;
at 150 ℃;
for 48h;
under 37503.8 Torr;
chemoselective reaction;
Green chemistry;
|
79 %Spectr. 84 %Spectr. |
|
With
potassium tert-butylate; hydrogen; [Ru(PtBuNNHBn)H(CO)Cl];
In
tetrahydrofuran;
at 19 - 24 ℃;
for 68h;
under 7500.75 Torr;
|
92 %Chromat. 89 %Chromat. |
-
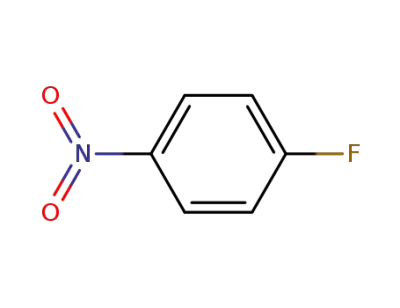
-
350-46-9,178603-76-4
4-Fluoronitrobenzene

-

-
100-51-6,185532-71-2
benzyl alcohol

-
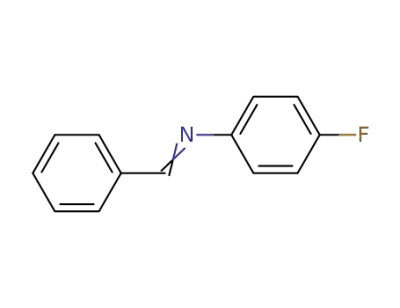
-
331-98-6,83306-62-1,83306-65-4
N-benzylidene-4-fluoroaniline

-
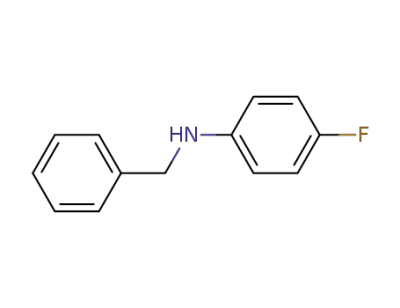
-
370-77-4
N-benzyl-p-fluoroaniline

-
-
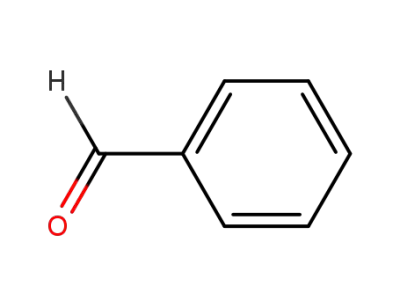
100-52-7
benzaldehyde

-
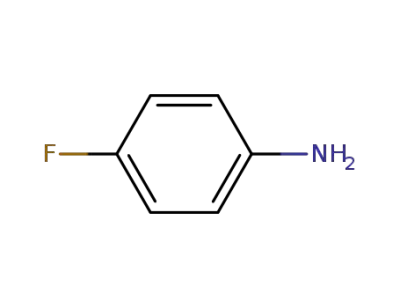
-
371-40-4
4-fluoroaniline
| Conditions | Yield |
|---|---|
|
With
potassium phosphate; hydrogen;
In
acetonitrile;
for 24h;
under 760.051 Torr;
chemoselective reaction;
Irradiation;
|
371-40-4 Upstream products
-
352-33-0
1-Chloro-4-fluorobenzene
-
456-22-4
4-Fluorobenzoic acid
-
100-65-2
N-Phenylhydroxylamine
-
98-95-3
nitrobenzene
371-40-4 Downstream products
-
780-05-2
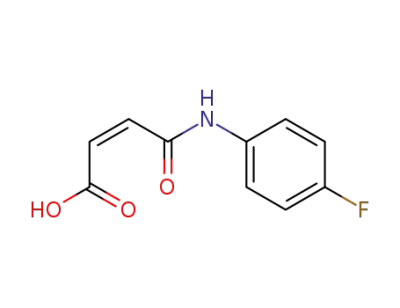
(Z)-4-((4-fluorophenyl)amino)-4-oxobut-2-enoic acid
-
348-45-8
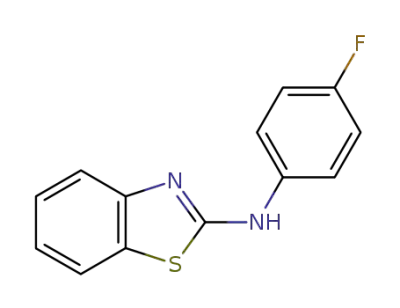
N-(4-fluorophenyl)benzo[d]thiazol-2-amine
-
2369-32-6
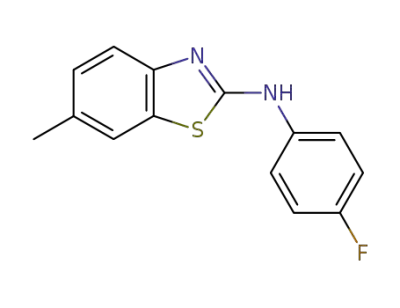
6-methyl-N-(4-fluorophenyl)benzo[d]thiazol-2-amine
-
494-26-8
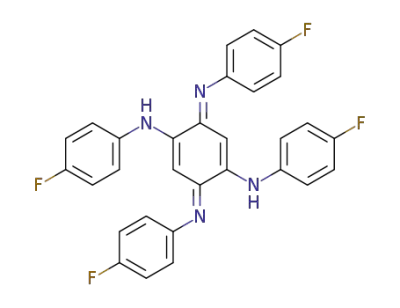
2,5-bis-(4-fluoro-anilino)-[1,4]benzoquinone-bis-(4-fluoro-phenylimine)
Relevant Products
-
1-Butyl-3-methylimidazolium tetrafluoroborate
CAS:174501-65-6
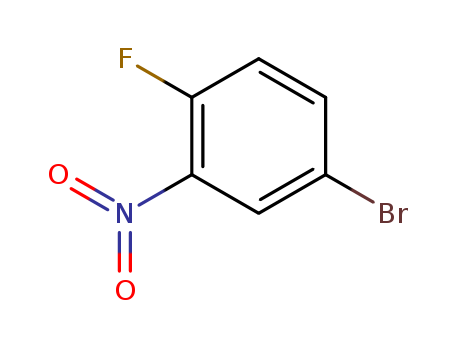
-
4-Bromo-1-fluoro-2-nitrobenzene
CAS:364-73-8
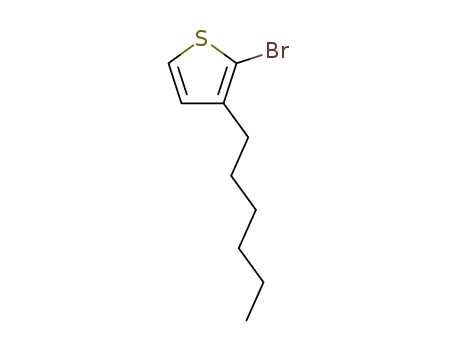
-
2-Bromo-3-hexylthiophene
CAS:69249-61-2