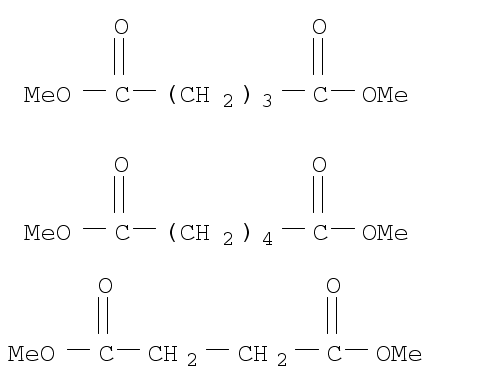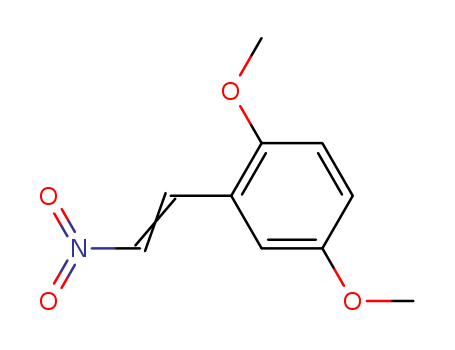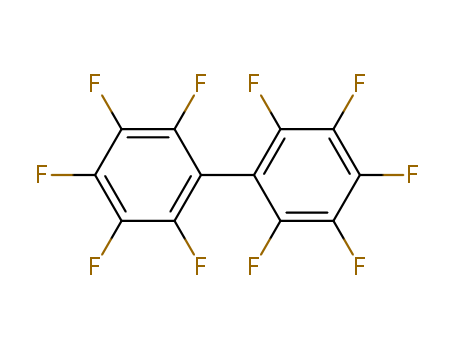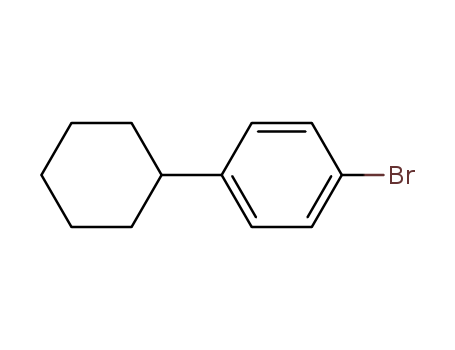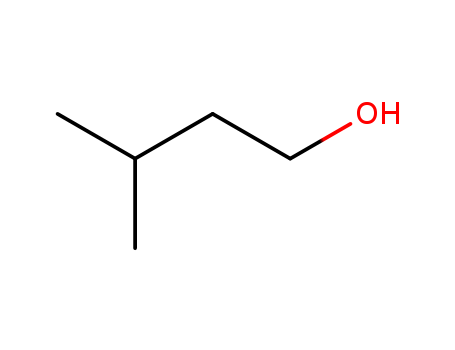
123-51-3
- Product Name:3-Methyl-1-butanol
- Molecular Formula:C5H12O
- Purity:99%
- Molecular Weight:88.1497
Product Details;
CasNo: 123-51-3
Molecular Formula: C5H12O
Appearance: colourless liquid
factory and supplier 123-51-3 3-Methyl-1-butanol in stock
- Molecular Formula:C5H12O
- Molecular Weight:88.1497
- Appearance/Colour:colourless liquid
- Vapor Pressure:2 mm Hg ( 20 °C)
- Melting Point:-117 °C
- Refractive Index:n20/D 1.407
- Boiling Point:131.2 °C at 760 mmHg
- PKA:>14 (Schwarzenbach et al., 1993)
- Flash Point:45.6 °C
- PSA:20.23000
- Density:0.809 g/cm3
- LogP:1.02480
3-Methyl-1-butanol(Cas 123-51-3) Usage
|
content analysis |
determined through non-polar column method of gas chromatography(GT-10-4). Toxicity? GRAS (FEMA). Use limits FEMA (mg/kg): soft drinks 17; cold drinks 7.6; candy 52; baked goods 24; pudding 46; gum 300; alcohol 100. Modest limit (FDA § 172.515, 2000). |
|
Production methods |
(1) This product naturally presents in the form of esters in strawberries, peppermint, lemongrass, eucalyptus oil and rum and so on. It can be synthesized by acid method or the hydroformylation of C4 alkenes. 3-methyl-1-butanol (85% in the fusel oil) can be obtained by chemical treatment and distillation separation of the fusel oil that is the side products form the alcohol fermentation of starch and sugar. (2) Derived from fusel oil fractionation. Pentane performs chlorination and hydrolysis reaction to form mixed alcohol, and then isoamyl alcohol can be derived from the mixed alcohol. |
|
Hazards & Safety Information |
Category???? Flammable liquids Toxic classification?? moderate toxic Acute Toxicity?? Oral-rat LD50: 1300 mg/kg; celiac-mouse LD50: 233 mg/kg Stimulation Data?? Skin-Rabbit 20mg/24hours Moderate; Eye-Rabbit 20mg/24hours Moderate Explosives hazardous characteristics?? Mix with air to be explosive Flammability hazard characteristics?? In case of fire, high temperature and oxidant flammable; combustion to release excitive smoke Storage and transportation characteristics?? Ventilation; Low temperature; dry; Separate storage with oxidizing agent Extinguishing agent? dry powder, dry sand, carbon dioxide, foam, 1211 extinguishing agent Occupational Standard? TLV-TWA 100 PPM (360 mg /m3); STEL 125 PPM (450 mg/m3) |
|
Production Methods |
3-Methyl-1-butanol is used as solvents for oils, fats, resins, and waxes; in the plastics industry in spinning polyacrylonitrile; and in manufacturing lacquers, chemicals, and pharmaceuticals. It is also used as flavoring agents and in fragrances. Industrial exposure is principally by the dermal contact and inhalation. |
|
Preparation |
Industrially prepared by rectification of fusel oil. |
|
Air & Water Reactions |
Highly flammable. Water soluble. |
|
Reactivity Profile |
3-Methyl-1-butanol attacks plastics [Handling Chemicals Safely, 1980. p. 236]. Mixtures with concentrated sulfuric acid and strong hydrogen peroxide may cause explosions. Mixing with hypochlorous acid in water or water/carbon tetrachloride solution can generate isoamyl hypochlorites, which may explode, particularly on exposure to sunlight or heat. Mixing with chlorine would also yield isoamyl hypochlorites [NFPA 491 M, 1991]. Base-catalysed reactions with isocyanates can occur with explosive violence [Wischmeyer,1969]. |
|
Hazard |
Moderate fire risk. Vapor is toxic and irritant. Explosive limits in air 1.2–9%. |
|
Health Hazard |
Very high vapor concentrations irritate eyes and upper respiratory tract. Continued contact with skin may cause irritation. |
|
Flammability and Explosibility |
Flammable |
|
Biochem/physiol Actions |
3-Methyl-1-butanol is a pentanol isomer useful in biofuels. It is used as a starting material for the production of isoamyl acetate, a flavoring agent applicable in the food industry. 3-Methyl-1-butanol shows anti-fungal action by inhibiting the hyphal formation and reducing biofilm formation in Candida albicans. It is also used in DNA extraction protocols. |
|
Potential Exposure |
(n-isomer); Suspected reprotoxic hazard, Primary irritant (w/o allergic reaction), (iso-, primary): Possible risk of forming tumors, Primary irritant (w/o allergic reaction), (sec-, active primary-, and other isomers) Primary irritant (w/o allergic reaction). Used as a solvent in organic synthesis and synthetic flavoring, pharmaceuticals, corrosion inhibitors; making plastics and other chemicals; as a flotation agent. The (n-isomer) is used in preparation of oil additives, plasticizers, synthetic lubricants, and as a solvent. |
|
Environmental fate |
Biological. Using the BOD technique to measure biodegradation, the mean 5-d BOD value (mM BOD/mM isoamyl alcohol) and ThOD were 4.46 and 59.5%, respectively (Vaishnav et al., 1987). Chemical/Physical. Isoamyl alcohol will not hydrolyze because it has no hydrolyzable functional group (Kollig, 1993). |
|
Shipping |
UN2811 Pentanols, Hazard Class: 3; Labels: 3- Flammable liquid. UN1987 Alcohols, n.o.s., Hazard Class: 3; Labels: 3-Flammable liquid. |
|
Purification Methods |
Dry the alcohol by heating with CaO and fractionally distilling, then heating with BaO and redistilling. Alternatively, boil it with concentrated KOH solution, wash it with dilute H3PO4, and dry it with K2CO3, then anhydrous CuSO4, before fractionally distilling it. If very dry alcohol is required, the distillate is refluxed with the appropriate alkyl phthalate or succinate as described for ethanol. It is separated from 2-methyl-1-butanol by fractional distillation, fractional crystallisation and preparative gas chromatography. [Beilstein 1 IV 1677.] |
|
Incompatibilities |
Forms an explosive mixture with air. Contact with strong oxidizers and hydrogen trisulfide may cause fire and explosions. Incompatible with strong acids. Violent reaction with alkaline earth metals forming hydrogen, a flammable gas. |
|
Waste Disposal |
Dissolve or mix the material with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber. All federal, state, and local environmental regulations must be observed. |
|
Chemical properties |
Colorless to pale yellow clear oily liquid. Apple brandy aroma and spicy flavor. Melting point:-117.2 °C. Boiling point: 130 °C. Relative density (d2525): 0.813. Refractive index (nD20): 1.4075. Vapors are toxic. Miscible in ethanol and ether. Slightly soluble in water. Natural products present in the form of esters in strawberries, peppermint, lemongrass, eucalyptus oil and rum and so on. |
|
Physical properties |
Clear, colorless liquid with a pungent odor. An odor threshold concentration of 1.7 ppbv was reported by Nagata and Takeuchi (1990). |
|
Definition |
ChEBI: An alkyl alcohol that is butan-1-ol substituted by a methyl group at position 3. |
|
Aroma threshold values |
Detection: 250 ppb to 4.1 ppm |
|
Taste threshold values |
Taste characteristics at 50 ppm: fusel, fermented, fruity, banana, ethereal and cognac |
|
General Description |
Colorless liquid with a mild, choking alcohol odor. Less dense than water, soluble in water. Hence floats on water. Produces an irritating vapor. |
InChI:InChI=1/C5H12O/c1-5(2)3-4-6/h5-6H,3-4H2,1-2H3
123-51-3 Relevant articles
Influence of Alkali Promoters in the Selective Hydrogenation of 3-Methyl-2-butenal over Ru/SiO2 Catalysts
Waghray, Akshay,Wang, Jian,Oukaci, Rachid,Blackmond, Donna G.
, p. 5954 - 5959 (1992)
The addition of potassium as a promoter ...
-
Wender et al.
, p. 5760 (1955)
-
Efficient and selective solvent-free homogeneous hydrogenation of aldehydes under mild reaction conditions using [RuCl2(dppb)(ampy)]
Angelini, Tommaso,Roseblade, Stephen,Zanotti-Gerosa, Antonio
, (2020)
The efficient, solvent-free homogeneous ...
Reactivity of 3-Methyl-Crotonaldehyde on Pt(111)
Birchem, T.,Pradier, C. M.,Berthier, Y.,Cordier, G.
, p. 503 - 510 (1994)
The reactivities of an α,β-unsaturated a...
Microwave-Induced Esterification Using Heterogeneous Acid Catalyst in a Low Dielectric Constant Medium
Kabza, Konrad G.,Chapados, Brian R.,Gestwicki, Jason E.,McGrath, Jessica L.
, p. 1210 - 1214 (2000)
-
Hydrogenation of 3-methyl-crotonaldehyde on the Pt(553) stepped surface: Influence of the structure and of preadsorbed tin
Birchem,Pradier,Berthier,Cordier
, p. 68 - 77 (1996)
The hydrogenation of 3-methyl-crotonalde...
-
Pedler
, p. 74 (1868)
-
-
Burger,Mosettig
, p. 1570 (1936)
-
Photocatalytic Regeneration of Nicotinamide Cofactors by Quantum Dot-Enzyme Biohybrid Complexes
Brown, Katherine A.,Wilker, Molly B.,Boehm, Marko,Hamby, Hayden,Dukovic, Gordana,King, Paul W.
, p. 2201 - 2204 (2016)
We report the characterization of biohyb...
Cyclodextrins as first and second sphere ligands for Rh(I) complexes of lower-rim PTA derivatives for use as catalysts in aqueous phase hydrogenation
Potier, Jonathan,Guerriero, Antonella,Menuel, Stéphane,Monflier, Eric,Peruzzini, Maurizio,Hapiot, Frédéric,Gonsalvi, Luca
, p. 74 - 78 (2015)
The rhodium complex [Rh(cod)Cl(N-tBuBzPT...
-
Baur,Schindler
, p. 1147 (1935)
-
Superior performance of a nanostructured platinum catalyst in water: Hydrogenations of alkenes, aldehydes and nitroaromatics
Maity, Prasenjit,Basu, Susmit,Bhaduri, Sumit,Lahiri, Goutam Kumar
, p. 1955 - 1962 (2007)
The hydrogenations of >C=CC=O and nitro ...
Infrared Spectroscopic Studies of the Adsorption and Reaction of 3-Methyl-2-butenal over Alkali-Promoted Ru/SiO2 Catalysts
Waghray, Akshay,Blackmond, Donna G.
, p. 6002 - 6006 (1993)
Flow reaction studies of the hydrogenati...
-
Josephson,v.Euler
, p. 54 (1924)
-
-
Greenwood,F.L.
, p. 1321 - 1324 (1964)
-
Structural insights into the cofactor-assisted substrate recognition of yeast methylglyoxal/isovaleraldehyde reductase Gre2
Guo, Peng-Chao,Bao, Zhang-Zhi,Ma, Xiao-Xiao,Xia, Qingyou,Li, Wei-Fang
, p. 1486 - 1492 (2014)
Saccharomyces cerevisiae Gre2 (EC1.1.1.2...
METHOD FOR PRODUCING BIO ALCOHOL FROM INTERMEDIATE PRODUCTS OF ANAEROBIC DIGESTION TANK
-
Paragraph 0057-0060; 0063; 0065-0066; 0068-0069; 0071, (2021/05/25)
The present invention relates to a metho...
Method for recycling byproducts in synthesis of diphenyl sulfide compound
-
Paragraph 0115; 0121-0123, (2021/03/30)
The invention provides a method for recy...
MANUFACTURING METHOD OF PENTYL ALCOHOL
-
Paragraph 0068; 0070; 0072-0075, (2021/08/05)
A method for preparing methyl butenoic a...
Chromium-Catalyzed Production of Diols From Olefins
-
Paragraph 0111, (2021/03/19)
Processes for converting an olefin react...
123-51-3 Process route
-
-
114429-15-1
C36H60O30*C5H12O

-
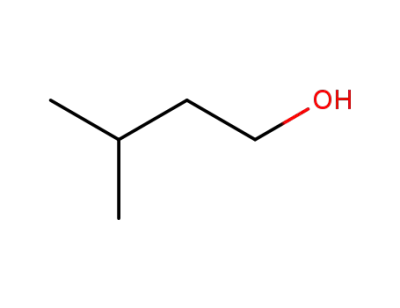
-
123-51-3
i-Amyl alcohol

-
-
10016-20-3
alpha cyclodextrin
| Conditions | Yield |
|---|---|
|
In
water;
at 25 ℃;
Equilibrium constant;
|
-
-
N-(3-methyl-butoxy)-benzamidine

-

-
123-51-3
i-Amyl alcohol

-

-
100-47-0
benzonitrile
| Conditions | Yield |
|---|---|
|
With
potassium hydroxide;
In
methanol;
at 15 ℃;
Electrochemical reaction;
|
81 % Chromat. |
123-51-3 Upstream products
-
75-21-8
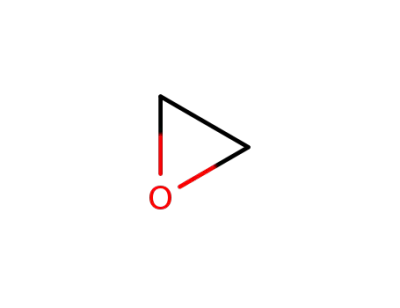
oxirane
-
920-39-8
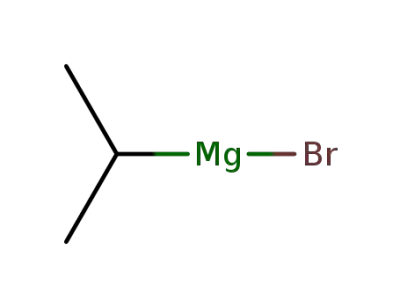
isopropylmagnesium bromide
-
3536-97-8
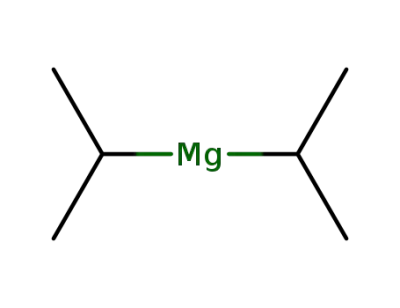
diisopropylmagnesium
-
766-15-4
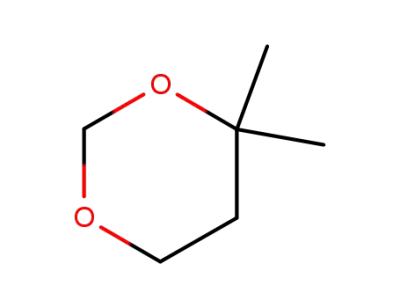
4,4-dimethyl-1,3-dioxane
123-51-3 Downstream products
-
859961-10-7
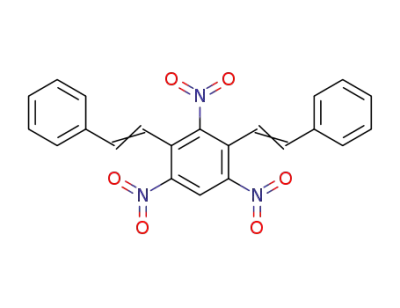
2,4,6-trinitro-1,3-bis(styryl)benzene
-
110-89-4
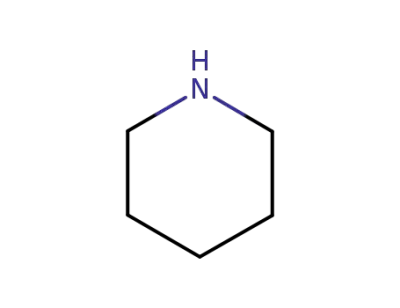
piperidine
-
4462-09-3
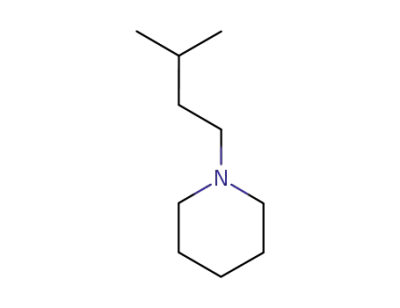
N-isopentylpiperidine
-
2308-18-1
isoamyl acetoacetate
Relevant Products
-
DIBASIC ESTER
CAS:95481-62-2
-
D-Alaninol
CAS:35320-23-1
-
5-Bromoindole-2-carboxylic acid
CAS:7254-19-5