64697-40-1
- Product Name:1-Methyl-3-octylimidazolium chloride
- Molecular Formula:C12H23ClN2
- Purity:99%
- Molecular Weight:230.781
Product Details;
CasNo: 64697-40-1
Molecular Formula: C12H23ClN2
Appearance: clear pale yellow to yellow viscous liquid
factory and manufacture 64697-40-1 1-Methyl-3-octylimidazolium chloride lonic liquid
- Molecular Formula:C12H23ClN2
- Molecular Weight:230.781
- Appearance/Colour:clear pale yellow to yellow viscous liquid
- Melting Point:12°C(lit.)
- Refractive Index:n20/D 1.51
- PSA:8.81000
- Density:1.01 g/cm3
- LogP:-0.32290
3-METHYL-1-OCTYLIMIDAZOLIUM CHLORIDE(Cas 64697-40-1) Usage
|
Conductivity |
0.09 mS/cm (30 °C) |
InChI:InChI=1/C12H23N2.ClH/c1-3-4-5-6-7-8-9-14-11-10-13(2)12-14;/h10-12H,3-9H2,1-2H3;1H/q+1;/p-1
64697-40-1 Relevant articles
Effect of alkyl groups in organic part of polyoxo-metalates based ionic liquids on properties of flame retardant polypropylene
Chen, Shengjiao,Wang, Chengle,Li, Juan
, p. 51 - 58 (2016)
The structure-property relationship of a...
Investigation of the role of ionic liquids in tuning the pK a values of some anionic indicators
Safavi,Abdollahi,Maleki,Zeinali
, p. 753 - 761 (2009)
The effect of imidazolium-based ionic li...
Deep desulfurization of light oil through extraction and oxidation processes using H2O2/tungstophosphoric acid in room-temperature ionic liquids
Chuang, Li-Lin,Huang, Jung-Fu,Lo, Wen-Hen,Wei, Guor-Tzo
, p. 324 - 330 (2012)
In this research, using room-temperature...
Modulating the Aggregation Behavior of 1-Methyl-3-Octylimidazolium Chloride by Alcohols in Aqueous Media
Pal, Amalendu,Yadav, Alka
, p. 1053 - 1062 (2016)
The self-organization and aggregation be...
Ionic liquids/water distribution ratios of some polycyclic aromatic hydrocarbons
Liu, Jing-Fu,Chi, Yu-Guang,Peng, Jin-Feng,Jiang, Gui-Bin,Joensson, Jan Ake
, p. 1422 - 1424 (2004)
By using the shake-flask procedure, the ...
Buffered chlorogallate(iii) ionic liquids and electrodeposition of gallium films
Seddon, Kenneth R.,Srinivasan, Geetha,Swad?ba-Kwa?ny, Ma?gorzata,Wilson, Anthony R.
, p. 4518 - 4526 (2013)
Buffering of Lewis acidic chlorometallat...
The Influence of Water and Metal Salt on the Transport and Structural Properties of 1-Octyl-3-methylimidazolium Chloride
Goujon, Nicolas,Byrne, Nolene,Walsh, Tiffany R.,Forsyth, Maria
, p. 420 - 425 (2015)
The addition of diluents to ionic liquid...
Opportunities for ionic liquids in recovery of biofuels
Fadeev,Meagher
, p. 295 - 296 (2001)
Room temperature ionic liquids have pote...
Ultrafast igniting, imidazolium based hypergolic ionic liquids with enhanced hydrophobicity
Bhosale, Vikas K.,Kulkarni, Prashant S.
, p. 1250 - 1258 (2017)
Exploring ultrafast igniting and hydroly...
Impact of imidazolium-based ionic liquids on the structure and stability of lysozyme
Satish, Lakkoji,Rana, Shubhasmin,Arakha, Manoranjan,Rout, Lipeeka,Ekka, Basanti,Jha, Suman,Dash, Priyabrat,Sahoo, Harekrushna
, p. 383 - 390 (2016)
Various types of water-miscible aprotic ...
Activation of hydrogen peroxide by the nitrate anion in micellar media
Schmidt, Fabian,Zehner, Bastian,Kaposi, Marlene,Drees, Markus,Mink, János,Korth, Wolfgang,Jess, Andreas,Cokoja, Mirza
supporting information, p. 1965 - 1971 (2021/03/26)
We present the activation of hydrogen pe...
The one-pot synthesis of butyl-1H-indol-3-alkylcarboxylic acid derivatives in ionic liquid as potent dual-acting agent for management of BPH
Chen, Kaixuan,Jiang, Zhenzhou,Liu, Shuwen,Xi, Baomin,Yang, Fubiao,Zeng, Li-Yan,Zeng, Yunong
, (2020/09/18)
Based on the SAR of both α1-AR antagonis...
Ionic liquid-assisted synthesis of Pt nano thin films at toluene–water interface: Enhanced CO tolerance in methanol fuel cells and adsorptive removal of p-nitrophenol from water
Sharfand, Saba Hamzepour,Hoseini, S. Jafar,Bahrami, Mehrangiz
, p. 483 - 497 (2018/06/26)
In this investigation, we report a one-p...
64697-40-1 Process route
-
-
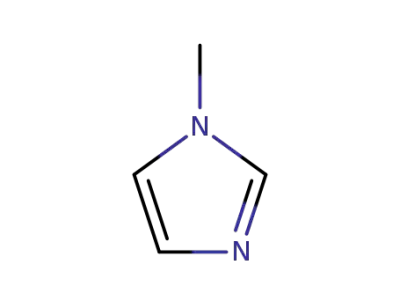
616-47-7
1-methyl-1H-imidazole

-
-
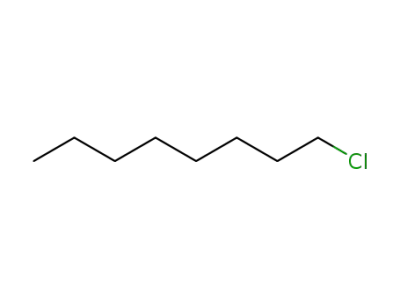
111-85-3
n-chlorooctane

-
-
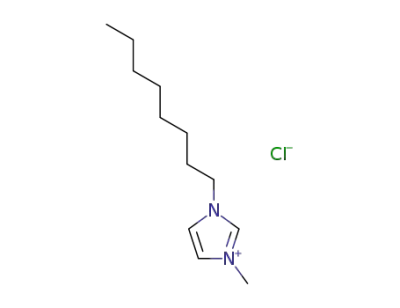
64697-40-1
1-methyl-3-octylimidazol-3-ium chloride
| Conditions | Yield |
|---|---|
|
In
neat (no solvent);
at 80 ℃;
for 24h;
Inert atmosphere;
|
100% |
|
at 150 ℃;
for 0.25h;
Microwave irradiation;
Neat (no solvent);
|
98% |
|
In
acetonitrile;
at 20 - 60 ℃;
Inert atmosphere;
|
95.92% |
|
1-methyl-1H-imidazole; n-chlorooctane;
at 20 ℃;
for 24h;
Inert atmosphere;
at 60 ℃;
for 216h;
Inert atmosphere;
|
95.9% |
|
at 100 - 125 ℃;
for 0.25h;
ultrasonic irradiation;
|
93% |
|
for 0.0583333h;
Microwave irradiation;
neat (no solvent);
|
89% |
|
at 80 ℃;
for 60h;
Inert atmosphere;
|
87% |
|
at 70 - 80 ℃;
|
86% |
|
at 80 ℃;
for 70h;
|
84.2% |
|
at 99.84 ℃;
for 29h;
|
80% |
|
In
cyclohexane; toluene;
at 70 ℃;
for 48h;
|
73.7% |
|
In
cyclohexane; toluene;
at 75 ℃;
for 48h;
|
72.4% |
|
In
cyclohexane; toluene;
at 75 ℃;
for 48h;
|
72.4% |
|
In
ethyl acetate;
for 72h;
Inert atmosphere;
Reflux;
|
71% |
|
In
ethyl acetate;
for 120h;
Reflux;
Inert atmosphere;
|
54% |
|
at 70 ℃;
for 72h;
|
|
|
at 69.85 ℃;
for 48h;
|
|
|
|
|
|
at 59.85 ℃;
for 48h;
|
|
|
at 80 ℃;
|
|
|
for 48h;
Heating;
|
|
|
at 100 ℃;
Inert atmosphere;
|
|
|
|
|
|
at 70 ℃;
for 72h;
|
|
|
In
tert-butyl methyl ether;
at 70 ℃;
|
|
|
Heating;
|
|
|
In
acetonitrile;
at 39.99 ℃;
for 72h;
Inert atmosphere;
|
|
|
In
acetonitrile;
Inert atmosphere;
|
|
|
In
acetonitrile;
at 80 ℃;
for 72h;
|
|
|
at 150 ℃;
for 0.5h;
Microwave irradiation;
Cooling;
neat (no solvent);
|
|
|
at 70 ℃;
|
|
|
In
acetonitrile;
at 199.99 ℃;
|
|
|
Thermodynamic data;
Heating;
|
|
|
at 70 ℃;
for 72h;
|
|
|
at 80 ℃;
for 48h;
|
|
|
In
acetonitrile;
for 48h;
Reflux;
|
|
|
at 80 ℃;
for 72h;
Inert atmosphere;
|
|
|
at 80 ℃;
for 24h;
Inert atmosphere;
|
|
|
at 69.84 ℃;
for 72h;
Inert atmosphere;
|
|
|
In
toluene;
at 180 ℃;
for 72h;
Inert atmosphere;
Cooling with ice;
|
|
|
In
acetonitrile;
at 70 ℃;
for 72h;
Inert atmosphere;
|
|
|
In
ethyl acetate;
for 24h;
Reflux;
|
|
|
at 90 ℃;
for 48h;
Inert atmosphere;
|
|
|
In
acetonitrile;
at 90 ℃;
for 72h;
|
|
|
at 69.99 ℃;
for 48h;
|
|
|
at 80 ℃;
Inert atmosphere;
|
|
|
at 70 ℃;
|
|
|
In
acetonitrile;
at 70 ℃;
for 6h;
|
-

-
616-47-7
1-methyl-1H-imidazole

-
-
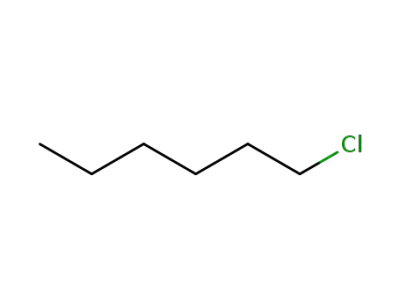
544-10-5
1-Chlorohexane

-

-
64697-40-1
1-methyl-3-octylimidazol-3-ium chloride
| Conditions | Yield |
|---|---|
|
at 100 ℃;
Inert atmosphere;
|
64697-40-1 Upstream products
-
616-47-7

1-methyl-1H-imidazole
-
111-85-3

n-chlorooctane
-
544-10-5

1-Chlorohexane
64697-40-1 Downstream products
-
244193-52-0
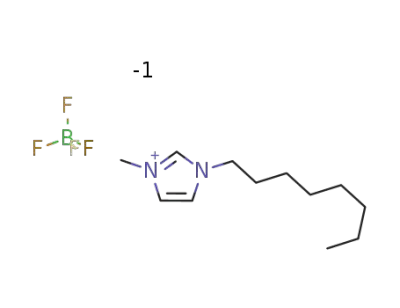
1-octyl-3-methylimidazolium tetrafluoroborate
-
111-85-3

n-chlorooctane
-
178631-04-4
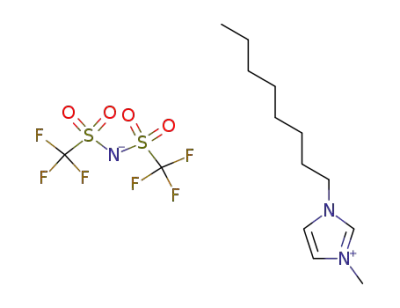
1-octyl-3-methylimidazolium bis(trifluoromethanesulfonyl)amide
-
403842-84-2
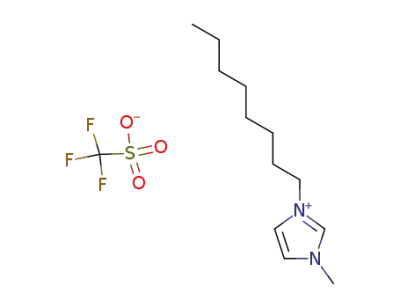
1-octyl-3-methyl-1H-imidazol-3-ium trifluoromethansulfonate
Relevant Products
-
1-Methylcyclopropanamine Hydrochloride
CAS:88887-87-0
-
5-Bromoindole-2-carboxylic acid
CAS:7254-19-5
-
8-[(2-hydroxybenzoyl)amino]octanoic acid
CAS:183990-46-7