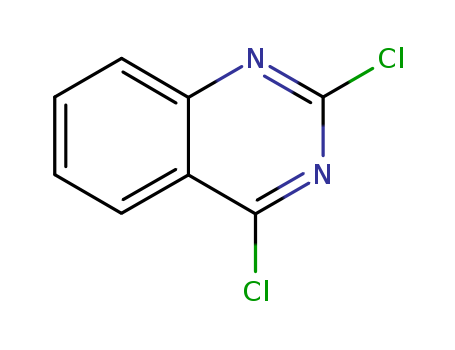
607-68-1
- Product Name:2,4-Dichloroquinazoline
- Molecular Formula:C8H4Cl2N2
- Purity:99%
- Molecular Weight:199.039
Product Details;
CasNo: 607-68-1
Molecular Formula: C8H4Cl2N2
Appearance: white crystalline powder
factory and supplier 607-68-1 2,4-Dichloroquinazoline in stock
- Molecular Formula:C8H4Cl2N2
- Molecular Weight:199.039
- Appearance/Colour:white crystalline powder
- Vapor Pressure:0.00173mmHg at 25°C
- Melting Point:117-121oC
- Refractive Index:1.531
- Boiling Point:273.3 °C at 760 mmHg
- PKA:-0.43±0.30(Predicted)
- Flash Point:145.4 °C
- PSA:25.78000
- Density:1.486 g/cm3
- LogP:2.93660
2,4-Dichloroquinazoline(Cas 607-68-1) Usage
InChI:InChI=1/C10H9NO2/c1-12-9-4-2-8(3-5-9)10-6-11-7-13-10/h2-7H,1H3
607-68-1 Relevant articles
Synthesis, characterization, crystal structure and cytotoxicity of 2,4-bis(selenomethyl)quinazoline
Plano, Daniel,Ibanez, Elena,Palop, Juan Antonio,Sanmartin, Carmen
, p. 1233 - 1240 (2011)
Organoselenium compounds have already be...
From triazolophthalazines to triazoloquinazolines: A bioisosterism-guided approach toward the identification of novel PCAF inhibitors with potential anticancer activity
El-Shershaby, Mohamed H.,Ghiaty, Adel,Bayoumi, Ashraf H.,Al-Karmalawy, Ahmed A.,Husseiny, Ebtehal M.,El-Zoghbi, Mona S.,Abulkhair, Hamada S.
, (2021)
Inhibition of PCAF bromodomain has been ...
Design, synthesis and anti-influenza A virus activity of novel 2,4-disubstituted quinazoline derivatives
Cen, Shan,Wang, Juxian,Wang, Minghua,Wang, Yucheng,Wang, Yujia,Zhang, Guoning,Zhu, Mei
, (2020)
Four 2,4-disubstituted quinazoline serie...
Design and discovery of new 1,2,4-triazolo[4,3-c]quinazolines as potential DNA intercalators and topoisomerase II inhibitors
Alesawy, Mohamed S.,Al-Karmalawy, Ahmed A.,Elkaeed, Eslam B.,Alswah, Mohamed,Belal, Ahmed,Taghour, Mohammed S.,Eissa, Ibrahim H.
, (2021)
A new series of 1,2,4-triazolo[4,3-c]qui...
Synthesis and antimicrobial activity of bis(azolyl)quinazoline-2,4-diamines
Rekha, Tamatam,Durgamma, Suram,Padmaja, Adivireddy,Padmavathi, Venkatapuram
, p. 1781 - 1792 (2017)
Abstract: Some new bis(azolylamino)- and...
Discovery of new coumarin substituted quinazolines as potential bioactive agents
Patel, Amit B.,Raval, Rinku M.
, p. 2163 - 2175 (2016)
In view of the biological importance of ...
[1,2,4]Triazolo[4,3-c]quinazoline and bis([1,2,4]triazolo)[4,3-a:4′,3′-c]quinazoline derived DNA intercalators: Design, synthesis, in silico ADMET profile, molecular docking and anti-proliferative evaluation studies
El-Adl, Khaled,Ibrahim, Mohamed-Kamal,Alesawy, Mohammed S.I.,Eissa, Ibrahim H.
, (2021)
In view of their DNA intercalation activ...
Design, synthesis, in silico ADMET, docking, and antiproliferative evaluations of [1,2,4]triazolo[4,3-c]quinazolines as classical DNA intercalators
Alesawy, Mohamed S.,Eissa, Ibrahim H.,El-Adl, Khaled,Ibrahim, Mohamed-Kamal
, (2022/01/13)
Eleven novel [1,2,4]triazolo[4,3-c]quina...
1,2,4-Triazolo[4,3-c]quinazolines: a bioisosterism-guided approach towards the development of novel PCAF inhibitors with potential anticancer activity
El-Shershaby, Mohamed H.,Ghiaty, Adel,Bayoumi, Ashraf H.,Ahmed, Hany E. A.,El-Zoghbi, Mona S.,El-Adl, Khaled,Abulkhair, Hamada S.
, p. 11136 - 11152 (2021/07/06)
Targeting PCAF with small inhibitor mole...
A Diverse Range of Hemozoin Inhibiting Scaffolds Act on Plasmodium falciparum as Heme Complexes
Openshaw, Roxanne,Maepa, Keletso,Benjamin, Stefan J.,Wainwright, Lauren,Combrinck, Jill M.,Hunter, Roger,Egan, Timothy J.
, p. 362 - 376 (2021/02/01)
A diverse series of hemozoin-inhibiting ...
607-68-1 Process route
-
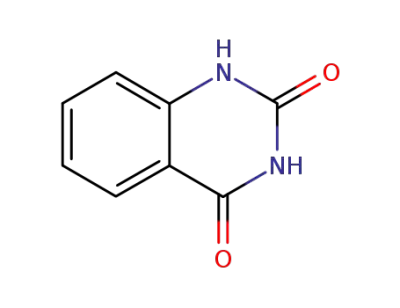
-
86-96-4
1,2,3,4-tetrahydro-quinazoline-2,4-dione

-
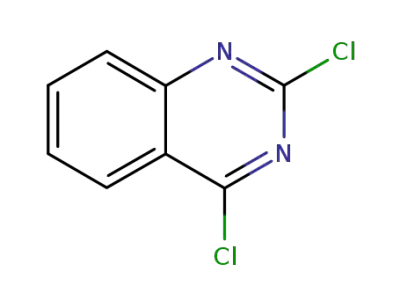
-
607-68-1
2,4-dichloroquinazoline
| Conditions | Yield |
|---|---|
|
With
trichlorophosphate;
at 115 ℃;
for 16h;
|
100% |
|
With
trichlorophosphate;
at 115 ℃;
for 16h;
|
100% |
|
With
trichlorophosphate;
for 18h;
Heating / reflux;
|
96% |
|
With
trichlorophosphate;
for 18h;
Heating / reflux;
|
96% |
|
With
trimethylamine; trichlorophosphate;
at 0 - 5 ℃;
for 7h;
|
95% |
|
With
triethylamine; trichlorophosphate;
at 120 ℃;
for 7h;
|
95% |
|
With
N,N-diethylaniline; trichlorophosphate;
for 5h;
Reflux;
|
92% |
|
With
N-ethyl-N,N-diisopropylamine; trichlorophosphate;
for 4h;
Reflux;
|
90% |
|
With
1-(1-methylpropyl)piperidine; trichlorophosphate;
at 80 - 85 ℃;
for 0.333333h;
|
89.4% |
|
With
N-ethyl-N,N-diisopropylamine; trichlorophosphate;
for 3h;
Reflux;
|
88% |
|
With
N,N-dimethyl-aniline; trichlorophosphate;
for 5h;
Reflux;
|
88% |
|
With
N,N-dimethyl-aniline; trichlorophosphate;
|
87% |
|
1,2,3,4-tetrahydro-quinazoline-2,4-dione;
With
trichlorophosphate;
In
toluene;
at 20 ℃;
for 0.25h;
Inert atmosphere;
With
tri-n-propylamine;
In
toluene;
at 55 - 110 ℃;
for 5h;
Inert atmosphere;
|
87.6% |
|
With
triethylamine; trichlorophosphate;
for 7h;
Reflux;
|
87% |
|
1,2,3,4-tetrahydro-quinazoline-2,4-dione;
With
trichlorophosphate;
at 20 ℃;
for 0.5h;
With
N,N-diethylaniline;
for 14h;
Reflux;
|
87% |
|
With
triethylamine; trichlorophosphate;
at 106 ℃;
for 18h;
Inert atmosphere;
|
87% |
|
With
N,N-dimethyl-aniline; trichlorophosphate;
for 7h;
Heating;
|
86% |
|
With
N,N-dimethyl-aniline; trichlorophosphate;
for 7h;
Heating;
|
86% |
|
With
N,N-dimethyl-aniline; trichlorophosphate;
for 7h;
Heating;
|
86% |
|
With
N,N-dimethyl-aniline; trichlorophosphate;
for 7h;
Heating / reflux;
|
86% |
|
With
N,N-dimethyl-aniline; trichlorophosphate;
for 7h;
Reflux;
|
80% |
|
With
trichlorophosphate;
In
DMF (N,N-dimethyl-formamide);
at 110 ℃;
for 17h;
|
79% |
|
1,2,3,4-tetrahydro-quinazoline-2,4-dione;
With
trichlorophosphate;
for 0.5h;
With
N,N-dimethyl-aniline;
for 7h;
Reflux;
|
79.6% |
|
With
trichlorophosphate;
at 20 ℃;
for 48.05h;
Heating / reflux;
|
76% |
|
1,2,3,4-tetrahydro-quinazoline-2,4-dione;
With
trichlorophosphate;
In
toluene;
at 50 - 105 ℃;
Inert atmosphere;
With
1,8-diazabicyclo[5.4.0]undec-7-ene;
In
toluene;
at 120 ℃;
for 24h;
Inert atmosphere;
|
75% |
|
1,2,3,4-tetrahydro-quinazoline-2,4-dione;
With
trichlorophosphate;
In
toluene;
at 50 ℃;
for 0.166667h;
Inert atmosphere;
With
1,8-diazabicyclo[5.4.0]undec-7-ene;
In
toluene;
at 105 - 120 ℃;
for 23h;
Inert atmosphere;
|
75% |
|
With
trichlorophosphate;
at 100 ℃;
for 24h;
|
75% |
|
With
N,N-dimethyl-aniline; trichlorophosphate;
at 115 ℃;
for 5h;
|
72% |
|
With
tri-n-propylamine; trichlorophosphate;
In
toluene;
at 110 ℃;
for 4h;
|
72% |
|
With
trichlorophosphate;
for 48h;
Reflux;
|
71% |
|
With
N,N-dimethyl-aniline; trichlorophosphate;
at 20 - 120 ℃;
for 24h;
|
68.6% |
|
With
N,N-dimethyl-aniline; trichlorophosphate;
at 120 ℃;
for 4h;
Inert atmosphere;
|
68% |
|
With
N,N-dimethyl-aniline; trichlorophosphate;
at 120 ℃;
for 24h;
|
68.6% |
|
With
triethylamine; trichlorophosphate;
for 7h;
Reflux;
|
67% |
|
With
trichlorophosphate;
In
N,N-dimethyl-aniline;
for 5h;
Reflux;
|
63% |
|
With
diethylamine; trichlorophosphate;
at 150 ℃;
for 0.25h;
Microwave irradiation;
|
62% |
|
With
N,N-dimethyl-aniline; trichlorophosphate;
for 5h;
Inert atmosphere;
Reflux;
|
61% |
|
With
N,N-dimethyl-aniline; trichlorophosphate;
at 0 ℃;
for 4h;
Reflux;
|
61% |
|
With
trichlorophosphate;
at 115 ℃;
Inert atmosphere;
Schlenk technique;
|
59% |
|
With
phosphorus pentachloride; trichlorophosphate;
In
N,N-dimethyl-aniline;
for 24h;
Reflux;
Inert atmosphere;
|
57% |
|
With
N,N-dimethyl-aniline; trichlorophosphate;
at 110 ℃;
for 24h;
|
56% |
|
With
N,N-dimethyl-aniline; trichlorophosphate;
for 5h;
Reflux;
|
52% |
|
With
phosphorus pentachloride; trichlorophosphate;
at 135 ℃;
|
51% |
|
With
trichlorophosphate;
N,N-dimethyl-aniline;
for 18h;
Reflux;
|
34% |
|
With
N,N-dimethyl-aniline; trichlorophosphate;
In
toluene;
for 18h;
Reflux;
|
34% |
|
With
N,N-dimethyl-aniline; trichlorophosphate;
for 3h;
Reflux;
|
34% |
|
1,2,3,4-tetrahydro-quinazoline-2,4-dione;
With
N,N-dimethyl-aniline; trichlorophosphate;
at 100 ℃;
for 16h;
With
water;
In
dichloromethane;
at 0 ℃;
|
33% |
|
With
N,N-dimethyl-aniline; trichlorophosphate;
at 100 ℃;
for 18h;
Inert atmosphere;
|
20% |
|
With
trichloroisocyanuric acid; triphenylphosphine;
In
toluene;
for 3h;
Heating;
|
11% |
|
With
trichlorophosphate;
|
|
|
With
N,N-dimethyl-aniline; trichlorophosphate;
|
|
|
With
N,N-dimethyl-aniline; trichlorophosphate;
for 5h;
Heating;
|
|
|
With
N,N-dimethyl-aniline; trichlorophosphate;
for 16h;
Heating;
|
|
|
In
ethyl acetate; N,N-dimethyl-aniline; trichlorophosphate;
|
|
|
With
trichlorophosphate;
In
hexane; N,N-dimethyl-aniline;
|
|
|
With
trichlorophosphate;
In
N,N-dimethyl-aniline;
|
|
|
With
trichlorophosphate;
In
hexane; N,N-dimethyl-aniline;
|
|
|
In
ice-water; trichlorophosphate;
|
|
|
With
N,N-dimethyl-aniline; trichlorophosphate;
at 130 ℃;
for 5h;
|
|
|
With
trichlorophosphate;
Reflux;
|
|
|
With
N,N-dimethyl-aniline; trichlorophosphate;
for 5h;
Reflux;
|
|
|
With
N,N-diethylaniline; trichlorophosphate;
for 96h;
Reflux;
|
|
|
With
trichlorophosphate;
Heating;
|
|
|
With
N,N-dimethyl-aniline; trichlorophosphate;
at 110 ℃;
for 3h;
|
|
|
With
trichlorophosphate;
at 20 ℃;
for 48h;
Reflux;
|
|
|
With
trichlorophosphate;
|
|
|
With
dmap; trichlorophosphate;
for 4h;
Reflux;
|
|
|
With
N,N-dimethyl-aniline; trichlorophosphate;
at 110 ℃;
for 3h;
Inert atmosphere;
|
|
|
With
N,N-dimethyl-formamide; trichlorophosphate;
for 48h;
Reflux;
|
|
|
With
N,N-dimethyl-aniline; trichlorophosphate;
for 14h;
Reflux;
|
|
|
With
trichlorophosphate;
In
N,N-dimethyl-aniline;
Reflux;
|
|
|
With
triethylamine; trichlorophosphate;
Reflux;
|
|
|
With
((1,3,5-triazine-2,4,6-triyl)tris(oxy))tris(triphenylphosphonium) chloride;
In
neat (no solvent);
at 170 - 180 ℃;
for 2h;
|
|
|
With
trichlorophosphate;
In
N,N-dimethyl-formamide;
|
|
|
With
N,N-dimethyl-aniline; trichlorophosphate;
for 5h;
Inert atmosphere;
Reflux;
|
14.5 g |
|
With
N,N-diethylaniline; trichlorophosphate;
In
acetonitrile;
Reflux;
|
|
|
With
N,N-dimethyl-aniline; trichlorophosphate;
Heating;
|
|
|
With
trichlorophosphate;
Alkaline conditions;
|
|
|
With
phosphorus pentachloride; trichlorophosphate;
at 120 ℃;
for 6h;
Inert atmosphere;
|
|
|
With
sodium hydrogencarbonate; trichlorophosphate;
In
hexane; N,N-dimethyl-aniline; benzene;
|
|
|
With
N-ethyl-N,N-diisopropylamine; trichlorophosphate;
In
toluene;
Reflux;
|
|
|
With
N-ethyl-N,N-diisopropylamine; trichlorophosphate;
In
acetonitrile;
at 100 ℃;
for 6h;
|
|
|
With
triethylamine; trichlorophosphate;
at 0 - 5 ℃;
for 7h;
Reflux;
|
|
|
With
trichlorophosphate;
for 7h;
Reflux;
|
|
|
With
trichlorophosphate;
In
(2S)-N-methyl-1-phenylpropan-2-amine hydrate;
|
510 mg (57%) |
|
With
triethylamine; trichlorophosphate;
for 7h;
Reflux;
|
-
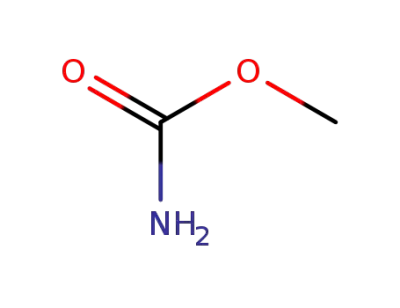
-
598-55-0
methyl carbamate

-
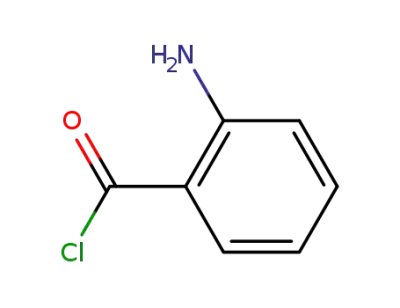
-
21563-73-5
2-aminobenzoyl chloride

-

-
607-68-1
2,4-dichloroquinazoline
| Conditions | Yield |
|---|---|
|
With
pyridine; tetrachloromethane; titanium(IV) oxide; vanadia; tungsten(VI) oxide; tin(IV) oxide;
In
N,N-dimethyl-formamide;
at 120 - 230 ℃;
for 32h;
under 2250.23 - 6000.6 Torr;
Solvent;
Temperature;
Pressure;
Reagent/catalyst;
|
99.1% |
607-68-1 Upstream products
-
607-19-2
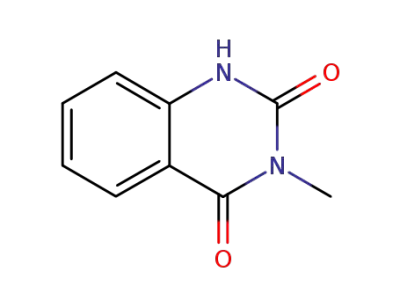
3-methyl-2,4(1H,3H)-quinazolinedione
-
86-96-4

1,2,3,4-tetrahydro-quinazoline-2,4-dione
-
120-94-5
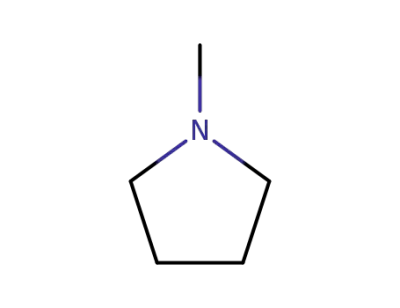
1-Methylpyrrolidine
-
607-69-2
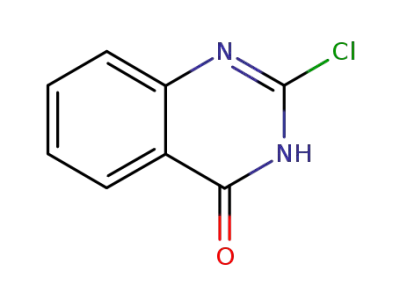
2-chloro-4(3H)-quinazolinone
607-68-1 Downstream products
-
77767-98-7
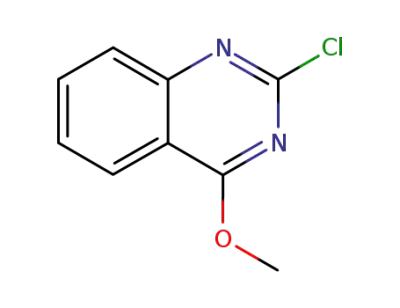
2-chloro-4-methoxyquinazoline
-
858235-57-1
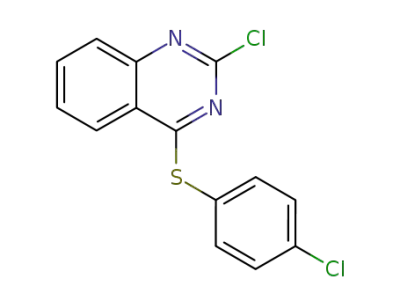
2-chloro-4-[(4-chlorophenyl)thio]quinazoline
-
611-65-4
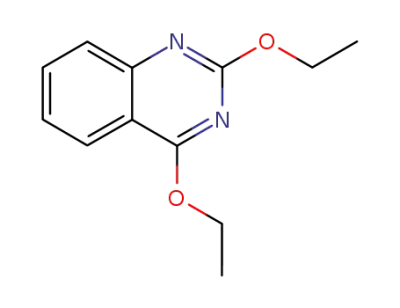
2,4-diethoxy-quinazoline
-
858027-20-0
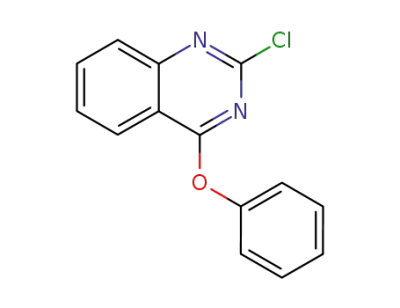
2-chloro-4-phenoxyquinazoline
Relevant Products
-
1-Methyl-3-octylimidazolium chloride
CAS:64697-40-1
-
5-Bromo-2-fluoroaniline
CAS:2924-09-6
-
1-Butyl-2,3-Dimethylimidazolium Chloride
CAS:98892-75-2