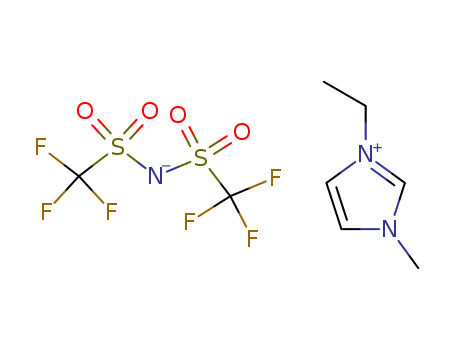
174899-83-3
- Product Name:1-Butyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide
- Molecular Formula:C10H15F6N3O4S2
- Purity:99%
- Molecular Weight:419.369
Product Details;
CasNo: 174899-83-3
Molecular Formula: C10H15F6N3O4S2
factory and manufacture 174899-83-3 1-Butyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide lonic liquid
- Molecular Formula:C10H15F6N3O4S2
- Molecular Weight:419.369
- Melting Point:1℃
- Refractive Index:n20/D 1.428
- Flash Point:>200 °C
- PSA:93.85000
- Density:1.44 g/cm3
- LogP:4.33360
1-BUTYL-3-METHYLIMIDAZOLIUM BIS(TRIFLUOR(Cas 174899-83-3) Usage
|
Conductivity |
3.41 mS/cm |
|
General Description |
1-Butyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide is a room temperature ionic liquid (RTIL). The solubility of alkane gases are less than the alkene gas in this IL. Its ability to solubilize CO2 is more when compared to 1-butyl-3-methylimidazolium dicyanamide. |
InChI:InChI=1/C8H15N2.C2F6NO4S2/c1-3-4-5-10-7-6-9(2)8-10;3-1(4,5)14(10,11)9-15(12,13)2(6,7)8/h6-8H,3-5H2,1-2H3;/q+1;-1
174899-83-3 Relevant articles
Understanding the behavior of mixtures of protic-aprotic and protic-protic ionic liquids: Conductivity, viscosity, diffusion coefficient and ionicity
Thawarkar, Sachin,Khupse, Nageshwar D.,Shinde, Dinesh R.,Kumar, Anil
, p. 986 - 994 (2019)
We have investigated the physicochemical...
Transition-state effects of ionic liquids in substitution reactions of PtII complexes
Weber, Christian F.,Puchta, Ralph,Van Eikema Hommes, Nico J. R.,Wasserscheid, Peter,Van Eldik, Rudi
, p. 6033 - 6038 (2005)
When normal is a surprise: Studies of a ...
First observation for dynamic solvent effect in ionic liquids
Kitaoka, Satoshi,Nobuoka, Kaoru,Miura, Junji,Ohga, Yasushi,Ishikawa, Yuichi
, p. 385 - 387 (2016)
We observed pressure effects on the rate...
The effects of an ionic liquid on unimolecular substitution processes: The importance of the extent of transition state solvation
Keaveney, Sinead T.,White, Benjamin P.,Haines, Ronald S.,Harper, Jason B.
, p. 2572 - 2580 (2016)
The reaction of bromodiphenylmethane and...
Extraction of uranium from aqueous solutions by using ionic liquid and supercritical carbon dioxide in conjunction
Wang, Joanna Shaofen,Sheaff, Chrystal N.,Yoon, Byunghoon,Addleman, R. Shane,Wai, Chien M.
, p. 4458 - 4463 (2009)
Uranyl ions [UO2]2+ in aqueous nitric ac...
Europium-based ionic liquids as luminescent soft materials
Tang, Sifu,Babai, Arash,Mudring, Anja-Verena
, p. 7631 - 7634 (2008)
Low melting, highly luminescent: [C3mim]...
Synthesis of CoPt nanorods in ionic liquids
Wang, Yong,Yang, Hong
, p. 5316 - 5317 (2005)
Cobalt platinum nanorods, hyperbranched ...
Binary Mixtures of Aprotic and Protic Ionic Liquids Demonstrate Synergistic Polarity Effect: An Unusual Observation
Thawarkar, Sachin,Khupse, Nageshwar D.,Kumar, Anil
, p. 210 - 221 (2020)
In this communication, we demonstrate th...
Evaluation of ionic liquids as electrolytes for vanadium redox flow batteries
Bahadori, L.,Boyd, R.,Nockemann, P.,Shafeeyan, M. S.,Warrington, A.
, (2020)
Non-aqueous redox flow batteries (NARFBs...
Notes on the asymmetric hydrogenation of methyl acetoacetate in neoteric solvents
Floris, Tomas,Kluson, Petr,Muldoon, Mark J.,Pelantova, Helena
, p. 279 - 287 (2010)
Asymmetric hydrogenation of methyl aceto...
Ambient lithium-SO2 batteries with ionic liquids as electrolytes
Xing, Huabin,Liao, Chen,Yang, Qiwei,Veith, Gabriel M.,Guo, Bingkun,Sun, Xiao-Guang,Ren, Qilong,Hu, Yong-Sheng,Dai, Sheng
, p. 2099 - 2103 (2014)
Li-SO2 batteries have a high energy dens...
Sonochemical synthesis of 0D, 1D, and 2D zinc oxide nanostructures in ionic liquids and their photocatalytic activity
Alammar, Tarek,Mudring, Anja-Verena
, p. 1796 - 1804 (2011)
Ultrasound synthesis of zinc oxide from ...
Facile and scalable synthesis of nanoporous materials based on poly(ionic liquid)s
Azcune, Itxaso,Garca, Ignacio,Carrasco, Pedro M.,Genua, Aratz,Tanczyk, Marek,Jaschik, Manfred,Warmuzinski, Krzysztof,Cabaero, Germn,Odriozola, Ibon
, p. 3407 - 3412 (2014)
A simple, fast, sustainable, and scalabl...
Physico-chemical properties of binary and ternary mixtures of ethyl acetate + ethanol + 1-butyl-3-methyl-imidazolium bis(trifluoromethylsulfonyl)imide at 298.15 K and atmospheric pressure
Andreatta, Alfonsina E.,Arce, Alberto,Rodil, Eva,Soto, Ana
, p. 371 - 383 (2010)
Densities, viscosities and refractive in...
Electrochemical and spectroscopic study of vanadyl acetylacetonate–ionic liquids interactions
Guglielmero,Langroudi, Mo. Meskinfam,Khatib, M. Al,de Oliveira, M. Aysla Costa,Mecheri,De Leo,Mezzetta,Guazzelli,Giglioli,Epifanio, A. D',Pogni,Chiappe,Pomelli
, (2021)
A panel of ionic liquids has been synthe...
Dissolution of the Rare-Earth Mineral Bastnaesite by Acidic Amide Ionic Liquid for Recovery of Critical Materials
Freiderich, John W.,Stankovich, Joseph J.,Luo, Huimin,Dai, Sheng,Moyer, Bruce A.
, p. 4354 - 4361 (2015)
Rare-earth elements provide the cornerst...
Synthesis of efficient SBA-15 immobilized ionic liquid catalyst and its performance for Friedel–Crafts reaction
He, Yibo,Zhang, Qinghua,Zhan, Xiaoli,Cheng, Dang-guo,Chen, Fengqiu
, p. 112 - 120 (2016)
Friedel–Crafts alkylation of benzene wit...
Rationalising the effects of ionic liquids on a nucleophilic aromatic substitution reaction
Hawker, Rebecca R.,Wong, Michaela J.,Haines, Ronald S.,Harper, Jason B.
, p. 6433 - 6440 (2017)
The nucleophilic aromatic substitution r...
Gluing Ionic Liquids to Oxide Surfaces: Chemical Anchoring of Functionalized Ionic Liquids by Vapor Deposition onto Cobalt(II) Oxide
Xu, Tao,Waehler, Tobias,Vecchietti, Julia,Bonivardi, Adrian,Bauer, Tanja,Schwegler, Johannes,Schulz, Peter S.,Wasserscheid, Peter,Libuda, Joerg
, p. 9072 - 9076 (2017)
Ionic liquids (IL) hold a great potentia...
Crystallization in ionic liquids: Synthesis, properties, and polymorphs of uranyl salts
Qu, Feng,Zhu, Qian-Qian,Liu, Chun-Li
, p. 6421 - 6432 (2014)
Crystallizations in uranyl-containing io...
The effects of using an ionic liquid as a solvent for a reaction that proceeds through a phenonium ion
Gilbert, Alyssa,Haines, Ronald S.,Harper, Jason B.
, (2021)
A unimolecular reaction that proceeds pr...
Temperature dependence of interactions between stable piperidine-1-yloxyl derivatives and a semicrystalline ionic liquid
Strehmel, Veronika,Rexhausen, Hans,Strauch, Peter,Strehmel, Bernd
, p. 2182 - 2190 (2010)
The stable 2,2,6,6-tetramethylpiperidine...
EuIII luminescence in a hygroscopic ionic liquid: Effect of water and evidence for a complexation process
Billard, Isabelle,Mekki, Soufiane,Gaillard, Clotilde,Hesemann, Peter,Moutiers, Gilles,Mariet, Clarisse,Labet, Alexandre,Buenzli, Jean-Claude G.
, p. 1190 - 1197 (2004)
The spectroscopic characteristics (excit...
Surface tension of binary mixtures of 1-alkyl-3-methyl-imidazolium bis(trifluoromethylsulfonyl)imide ionic liquids with alcohols
Andreatta, Alfonsina E.,Rodil, Eva,Arce, Alberto,Soto, Ana
, p. 404 - 420 (2014)
New experimental surface tension data ha...
ZIF-8-porous ionic liquids for the extraction of 2,2,3,3-tetrafluoro-1-propanol and water mixture
Wang, Zenghui,Zhao, Pingping,Wu, Jimin,Gao, Jun,Zhang, Lianzheng,Xu, Dongmei
supporting information, p. 8557 - 8562 (2021/05/26)
The design of stable ionic liquids (ILs)...
174899-83-3 Process route
-
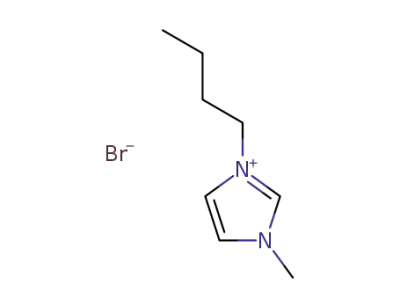
-
85100-77-2
1-n-butyl-3-methylimidazolim bromide

-
-
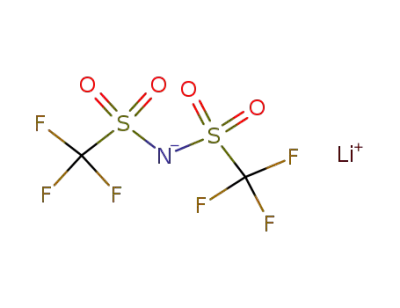
90076-65-6
bis(trifluoromethane)sulfonimide lithium

-
-
174899-83-3
1-butyl-3-methylimidazolium trifluoromethanesulfonimide
| Conditions | Yield |
|---|---|
|
In
water;
at 20 ℃;
for 2h;
|
97% |
|
In
dichloromethane; water;
|
96% |
|
In
water;
at 20 ℃;
for 24h;
|
95% |
|
In
water;
at 70 ℃;
|
94% |
|
|
94% |
|
In
water;
at 20 ℃;
|
92% |
|
With
water;
In
dichloromethane;
|
91% |
|
In
dichloromethane; water;
at 20 ℃;
for 16h;
|
91.8% |
|
In
water;
at 20 ℃;
for 18h;
|
90% |
|
In
water;
at 20 ℃;
for 18h;
|
90% |
|
In
water;
|
87.5% |
|
In
water;
at 20 ℃;
for 17h;
|
87% |
|
In
water;
at 20 ℃;
for 16h;
|
86% |
|
In
water;
at 20 ℃;
for 1h;
|
85% |
|
In
dichloromethane; water;
Kinetics;
|
83% |
|
In
dichloromethane;
for 48h;
|
81% |
|
In
dichloromethane; water;
at 40 ℃;
|
75% |
|
In
dichloromethane;
at 20 ℃;
for 24h;
|
60% |
|
at 60 - 70 ℃;
|
|
|
In
water;
at 20 ℃;
|
|
|
|
|
|
In
water;
at 20 ℃;
for 3h;
Product distribution / selectivity;
Electrochemical reaction;
|
|
|
at 100 ℃;
for 0.25h;
Microwave irradiation;
Neat (no solvent);
|
|
|
In
water;
for 3h;
|
|
|
In
water;
at 20 ℃;
for 24h;
Inert atmosphere;
Reflux;
|
|
|
In
water;
|
|
|
In
water;
|
|
|
In
water;
|
|
|
In
water;
at 70 ℃;
for 24h;
pH=6;
|
|
|
In
water;
|
|
|
at 80 ℃;
for 24h;
Inert atmosphere;
|
|
|
In
acetone;
at 20 ℃;
for 24h;
Inert atmosphere;
|
|
|
In
water;
at 20 ℃;
for 2h;
|
|
|
In
water;
at 20 ℃;
for 24h;
|
44.8 g |
|
for 24h;
Inert atmosphere;
|
|
|
In
water;
at 20 ℃;
for 24h;
|
|
|
In
dichloromethane; water;
at 20 ℃;
for 1h;
|
|
|
In
water;
at 20 ℃;
|
|
|
In
water;
at 20 ℃;
|
|
|
In
water;
|
|
|
In
acetonitrile;
at 20 ℃;
for 24h;
|
-
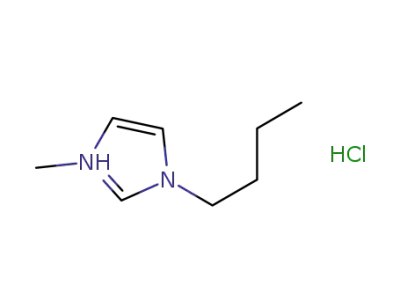
-
1-butyl-3-methylimidazolium chloride

-

-
174899-83-3
1-butyl-3-methylimidazolium trifluoromethanesulfonimide
| Conditions | Yield |
|---|---|
|
With
bis(trifluoromethane)sulfonimide lithium;
In
acetone;
for 24h;
|
95% |
174899-83-3 Upstream products
-
79917-90-1
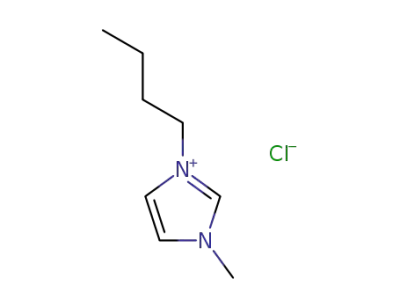
1-butyl-3-methylimidazolium chloride
-
90076-65-6

bis(trifluoromethane)sulfonimide lithium
-
85100-77-2

1-n-butyl-3-methylimidazolim bromide
-
342789-81-5
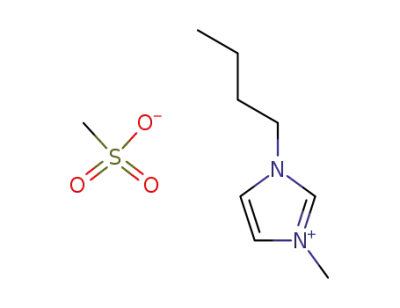
1-n-butyl-3-methylimidazolium methanesulfonate
174899-83-3 Downstream products
-
37595-74-7
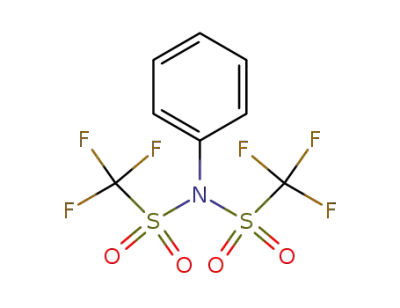
N,N-phenylbistrifluoromethane-sulfonimide
-
952128-74-4
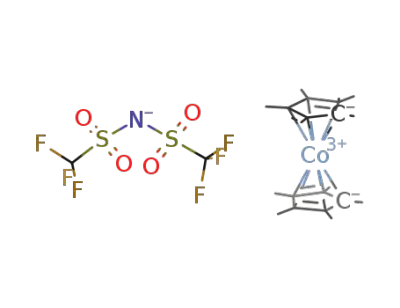
[(C5Me5)2Co(III)][NTf2]
-
616-47-7
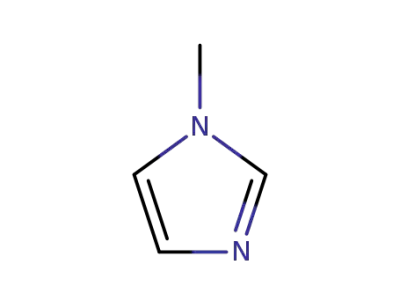
1-methyl-1H-imidazole
-
4316-42-1
1-Butylimidazole
Relevant Products
-
1-ethyl-3-methylimidazolium bis[(trifluoromethyl)sulfonyl]imide
CAS:174899-82-2
-
6-Aminonicotinic acid
CAS:3167-49-5
-
4-Bromo-1-chloro-2-fluorobenzene
CAS:60811-18-9