79917-90-1
- Product Name:1-Butyl-3-methylimidazolium chloride
- Molecular Formula:C8H15ClN2
- Purity:99%
- Molecular Weight:174.673
Product Details;
CasNo: 79917-90-1
Molecular Formula: C8H15ClN2
Appearance: colorless to straw yellow liquid
factory and manufacture 79917-90-1 1-Butyl-3-methylimidazolium chloride lonic liquid
- Molecular Formula:C8H15ClN2
- Molecular Weight:174.673
- Appearance/Colour:colorless to straw yellow liquid
- Melting Point:~70 °C
- Flash Point:192 °C
- PSA:8.81000
- Density:d25 1.08 (dried)
- LogP:-1.88330
1-Butyl-3-methylimidazolium chloride(Cas 79917-90-1) Usage
|
General Description |
1-Butyl-3-methylimidazolium chloride ([BMIM]Cl) is an organic chloride salt used as a precursor in synthesizing chloroindate(III) ionic liquids, which serve as recyclable and efficient media for Friedel-Crafts acylation reactions. These ionic liquids overcome limitations of traditional Lewis acid catalysts by providing a stable, reusable system for producing aromatic ketones. |
InChI:InChI=1/C8H15N2/c1-3-4-5-10-7-6-9(2)8-10/h6-8H,3-5H2,1-2H3/q+1
79917-90-1 Relevant articles
Experimental measurement and modeling of vapor-liquid equilibrium for the ternary system water + ethanol + 1-Butyl-3-methylimidazolium chloride
Geng, Wei,Zhang, Lianzhong,Deng, Dongshun,Ge, Yun,Ji, Jianbing
, p. 1679 - 1683 (2010)
Vapor-liquid equilibrium (VLE) data were...
Various metal organic frameworks combined with imidazolium, quinolinum and benzothiazolium ionic liquids for removal of three antibiotics from water
Yohannes, Alula,Li, Jing,Yao, Shun
, (2020/10/02)
In this research, imidazolium, quinolinu...
Method for synthesizing antioxygen 1076
-
Paragraph 0020; 0021, (2017/07/21)
The invention belongs to the technical f...
Recyclable zinc (II) ionic liquid catalyzed synthesis of azides by direct azidation of alcohols using trimethylsilylazide at room temperature
Singh, Ashima,Singh, Harjinder,Khurana
, p. 2498 - 2502 (2017/05/31)
A new efficient method has been reported...
A New Mode of Operation of Pd-NHC Systems Studied in a Catalytic Mizoroki-Heck Reaction
Astakhov, Alexander V.,Khazipov, Oleg V.,Chernenko, Andrey Yu.,Pasyukov, Dmitry V.,Kashin, Alexey S.,Gordeev, Evgeniy G.,Khrustalev, Victor N.,Chernyshev, Victor M.,Ananikov, Valentine P.
, p. 1981 - 1992 (2017/06/14)
Metal complexes bearing N-heterocyclic c...
79917-90-1 Process route
-
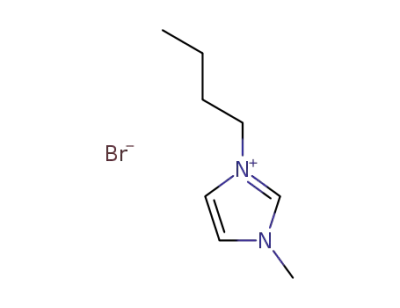
-
85100-77-2
1-n-butyl-3-methylimidazolim bromide

-
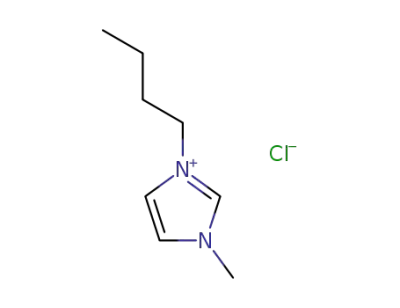
-
79917-90-1
1-butyl-3-methylimidazolium chloride
| Conditions | Yield |
|---|---|
|
With
hydrogenchloride;
In
acetone;
at 40 ℃;
for 10h;
|
-
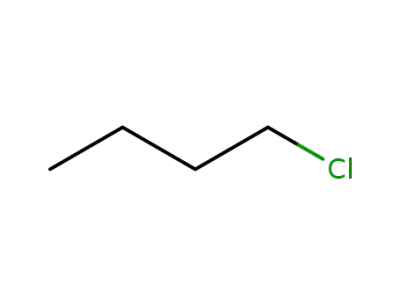
-
109-69-3
n-Butyl chloride

-

-
79917-90-1
1-butyl-3-methylimidazolium chloride
| Conditions | Yield |
|---|---|
|
With
1-methyl-1H-imidazole;
|
98% |
79917-90-1 Upstream products
-
616-47-7
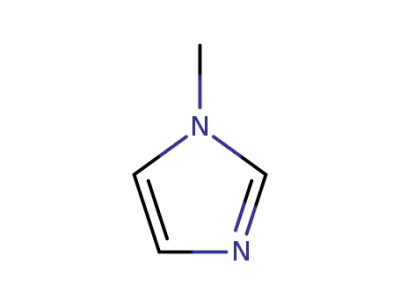
1-methyl-1H-imidazole
-
109-65-9
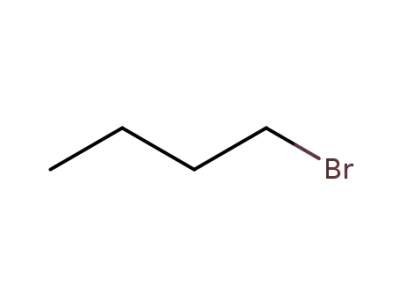
1-bromo-butane
-
109-69-3

n-Butyl chloride
-
497144-87-3
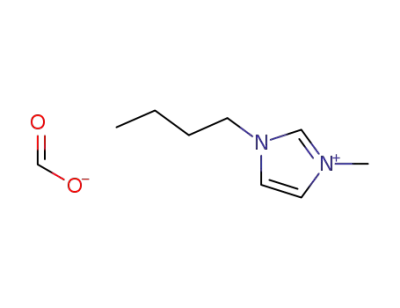
1-butyl-3-methylimidazolium formate
79917-90-1 Downstream products
-
401788-98-5
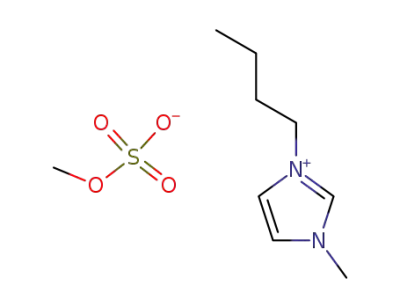
1-butyl-3-methylimidazolium methylsulfate
-
174899-66-2
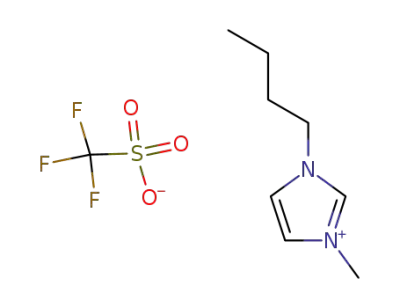
1-(n-butyl)-3-methylimidazolium triflate
-
174899-83-3
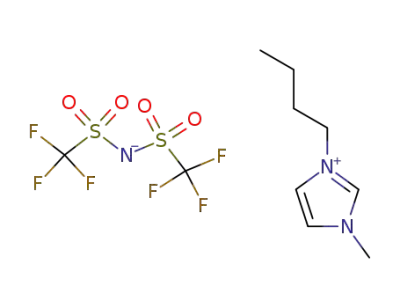
1-butyl-3-methylimidazolium trifluoromethanesulfonimide
-
448245-52-1
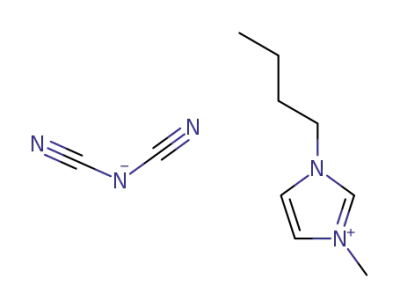
1-buthyl-3-methylimidazolium dicyanamide
Relevant Products
-
1-Methyl-3-octylimidazolium chloride
CAS:64697-40-1
-
1H,1H,5H-Octafluoropentyl Methacrylate
CAS:355-93-1
-
Methyl 2-bromothiophene-3-carboxylate
CAS:76360-43-5