348-54-9
- Product Name:2-Fluoroaniline
- Molecular Formula:C6H6FN
- Purity:99%
- Molecular Weight:111.119
Product Details;
CasNo: 348-54-9
Molecular Formula: C6H6FN
Appearance: Clear colorless to brown liquid
factory and supplier 348-54-9 2-Fluoroaniline in stock
- Molecular Formula:C6H6FN
- Molecular Weight:111.119
- Appearance/Colour:Clear colorless to brown liquid
- Vapor Pressure:1 hPa (20 °C)
- Melting Point:-29 °C
- Refractive Index:1.5420
- Boiling Point:182.499 °C at 760 mmHg
- PKA:3.2(at 25℃)
- Flash Point:60 °C
- PSA:26.02000
- Density:1.158 g/cm3
- LogP:1.98910
2-Fluoroaniline(Cas 348-54-9) Usage
|
Synthesis Reference(s) |
Tetrahedron Letters, 25, p. 3415, 1984 DOI: 10.1016/S0040-4039(01)91034-2 |
|
Air & Water Reactions |
Water soluble. |
|
Reactivity Profile |
2-Fluoroaniline is a base. Neutralizes acids to form salts plus water in an exothermic reaction. May be incompatible with isocyanates, halogenated organics, peroxides, phenols (acidic), epoxides, anhydrides, and acid halides. Flammable gaseous hydrogen is generated by amines in combination with strong reducing agents, such as hydrides. |
|
Health Hazard |
Inhalation or ingestion causes bluish tint to fingernails, lips and ears indicative of cyanosis; headache, drowsiness, and nausea, followed by unconsciousness. Liquid can be absorbed through skin and cause similar symptoms. Contact with eyes causes irritation. |
|
Fire Hazard |
Special Hazards of Combustion Products: Irritating and toxic hydrogen fluoride and oxides of nitrogen may form in fires. |
|
Metabolic pathway |
2-Fluoro and 3-fluoroanilines are preferentially hydroxylated at the para-position, and 4-fluoroaniline is both p- and o-hydroxylated to a significant extent by rat liver microsomes and is not accompanied with an NIH shift to give 4-hydroxyl-3-fluoroaniline. |
|
Application |
2-Fluoroaniline can be used to synthesize thermosensitive dye FH-102, which is a black thermosensitive dye with wide application and excellent performance. The dye can be used in the production of terminal output printing paper of electronic computer, EEG recording paper, telephone fax paper and transparent thermal film for oil field. |
|
Definition |
ChEBI: 2-fluoroaniline is a derivative of aniline in which the hydrogen at position 2 has been substituted by fluorine. It is used as a pharmaceutical intermediate It is a primary arylamine and a fluoroaniline. |
|
General Description |
Clear liquid with a mild sweet odor. Sinks in and mixes slowly with water. |
InChI:InChI=1/C6H6FN/c7-5-3-1-2-4-6(5)8/h1-4H,8H2
348-54-9 Relevant articles
Synthesis of Pd/SBA-15 catalyst employing surface-bonded vinyl as a reductant and its application in the hydrogenation of nitroarenes
Duan, Ying,Zheng, Min,Li, Dongmi,Deng, Dongsheng,Wu, Cuicui,Yang, Yanliang
, p. 3443 - 3449 (2017)
The Pd/SBA-15 catalyst was synthesised t...
Photocatalytic hydrogenation of nitroarenes: supporting effect of CoOx on TiO2 nanoparticles
Amanchi, Srinivasa Rao,Ashok Kumar,Lakshminarayana, Bhairi,Satyanarayana,Subrahmanyam
, p. 748 - 754 (2019)
Cobalt oxide visible light-active photo-...
Role of Charge Transfer in Nucleophilic Substitution Reactions in Clusters of 1-Fluoro-n-chlorobenzene Cations with Ammonia Molecules
Riehn, C.,Lahmann, C.,Brutschy, B.
, p. 3626 - 3632 (1992)
The reaction behavior of mixed clusters ...
Aminal-based Hypercrosslinked Polymer Modified with Small Palladium Nanoparticles for Efficiently Catalytic Reduction of Nitroarenes
Xu, Dan,Wang, Fushan,Yu, Guiqin,Zhao, Hong,Yang, Jing,Yuan, Man,Zhang, Xiaoyun,Dong, Zhengping
, p. 4569 - 4577 (2018)
Fabrication of heterogeneous catalysts w...
A Radical-Mediated Approach to the Total Synthesis of Fluorinated Marinoquinoline A and Related Tricyclic and Tetracyclic Congeners
Patel, Bhaven,Hilton, Stephen T.
, p. 79 - 83 (2015)
A radical-mediated approach to the core ...
Highly selective hydrogenation of halonitroaromatics to aromatic haloamines by ligand modified Ni-based catalysts
Lu, Chun Shan,Lv, Jing Hui,Ma, Lei,Zhang, Qun Feng,Feng, Feng,Li, Xiao Nian
, p. 545 - 548 (2012)
Ligand modification of Ni-based catalyst...
PVA-encapsulated Palladium Nanoparticles: Eco-friendly and Highly Selective Catalyst for Hydrogenation of Nitrobenzene in Aqueous Medium
Wang, Xiaoyan,Huang, Changru,Li, Xiaohao,Xie, Congxia,Yu, Shitao
, p. 2266 - 2272 (2019)
In aqueous medium without any other addi...
Synthesis of Pt nanocatalysts for selective hydrogenation of ortho-halogenated nitrobenzene
Xie, Ruigang,Cao, Xueqin,Pan, Yue,Gu, Hongwei
, p. 1051 - 1055 (2015)
Monodisperse Pt nanoparticles (NPs) were...
Minimization of Back-Electron Transfer Enables the Elusive sp3 C?H Functionalization of Secondary Anilines
Zhao, Huaibo,Leonori, Daniele
supporting information, p. 7669 - 7674 (2021/03/08)
Anilines are some of the most used class...
Ligand compound for copper catalyzed aryl halide coupling reaction, catalytic system and coupling reaction
-
Paragraph 0111-0119, (2021/05/29)
The invention provides a ligand compound...
In situcreation of multi-metallic species inside porous silicate materials with tunable catalytic properties
Liu, Yang-Yang,Wu, Chuan-De,Zhan, Guo-Peng
supporting information, p. 6185 - 6188 (2021/06/30)
Porous metal silicate (PMS) material PMS...
A suitable modified palladium immobilized on imidazolium supported ionic liquid catalysed transfer hydrogenation of nitroarenes
Atheeswari, Alagudurai,Kanimozhi, Nallusamy,Karthikeyan, Parasuraman,Shanmugapriya, Ramasamy
, (2021/06/28)
The first well-defined modified palladiu...
348-54-9 Process route
-
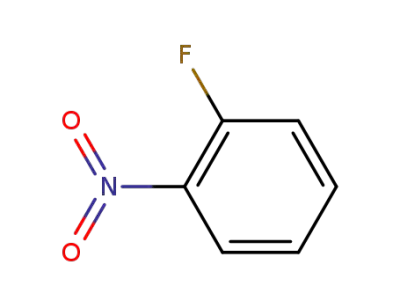
-
1493-27-2,127723-77-7
ortho-nitrofluorobenzene

-
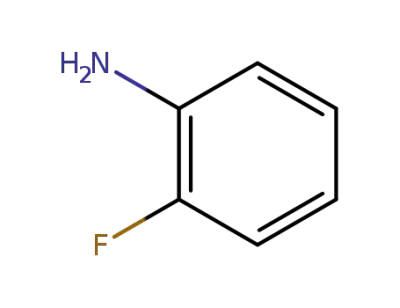
-
348-54-9
2-Fluoroaniline

-
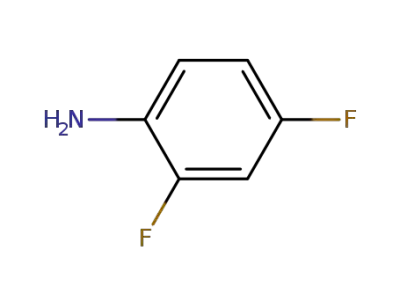
-
367-25-9,76563-56-9
2,4-difluorophenylamine
| Conditions | Yield |
|---|---|
|
With
bismuth; hydrogen fluoride;
In
dichloromethane;
Yields of byproduct given;
|
65% |
-
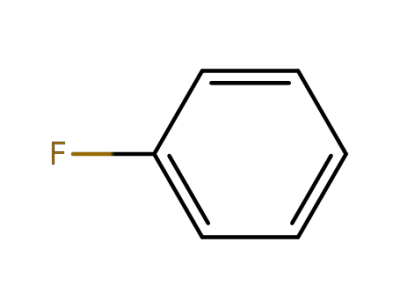
-
462-06-6
fluorobenzene

-
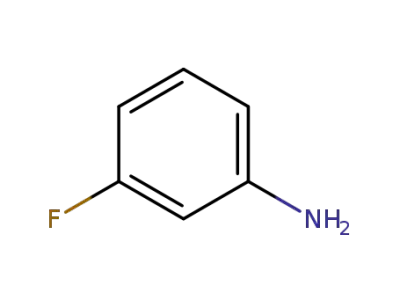
-
372-19-0
meta-fluoroaniline

-

-
348-54-9
2-Fluoroaniline

-
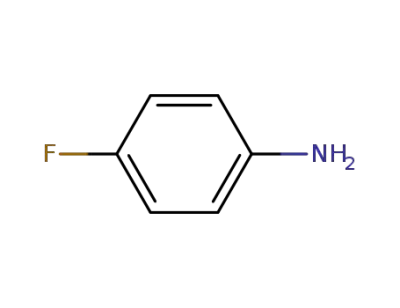
-
371-40-4
4-fluoroaniline
| Conditions | Yield |
|---|---|
|
With
hydroxylamine-O-sulfonic acid;
iron(II) sulfate;
In
water; acetic acid;
at 40 ℃;
for 2h;
Yield given. Yields of byproduct given;
|
|
|
With
hydroxylamine-O-sulfonic acid;
iron(II) sulfate;
In
water; acetic acid;
at 40 ℃;
for 2h;
Product distribution;
Mechanism;
k(C6H5F)/k(C6H6);
|
|
|
With
sodium azide; boron trifluoride monohydrate;
at 55 ℃;
for 14h;
Overall yield = 48 %;
Sealed tube;
|
348-54-9 Upstream products
-
348-51-6
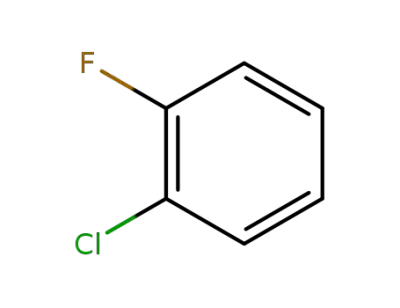
1-chloro-2-fluorobenzene
-
445-28-3
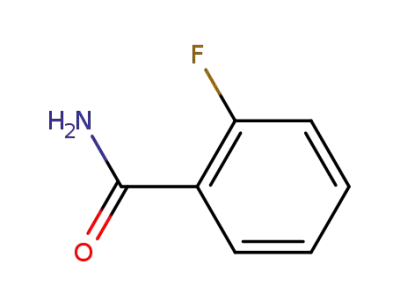
2-fluorobenzamide
-
446-23-1
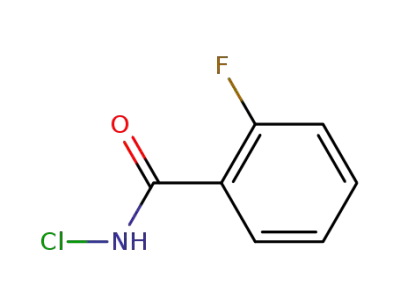
N-chloro-2-fluorobenzamide
-
1493-27-2

ortho-nitrofluorobenzene
348-54-9 Downstream products
-
368-05-8
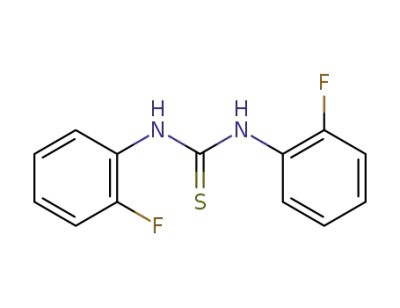
sym N,N′-di(2-fluorophenyl)thiourea
-
568-95-6
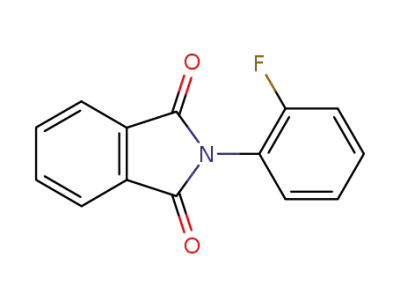
2-(2-fluorophenyl)isoindoline-1,3-dione
-
349-24-6
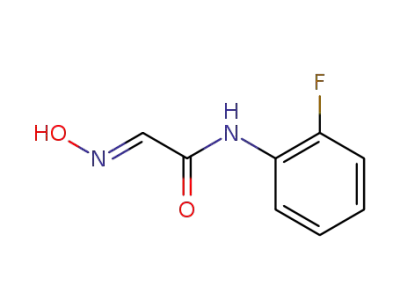
N-(2-Fluorophenyl)-2-hydroxyiminoacetamide
-
575-24-6
N-(2-fluorophenyl)-1-naphthylamine
Relevant Products
-
1-Butyl-3-methylimidazolium tetrafluoroborate
CAS:174501-65-6
-
(R)-(-)-2-(N-Methylamino)-3-phenylpropan-1-ol
CAS:102339-81-1
-
3-Bromoaniline
CAS:591-19-5