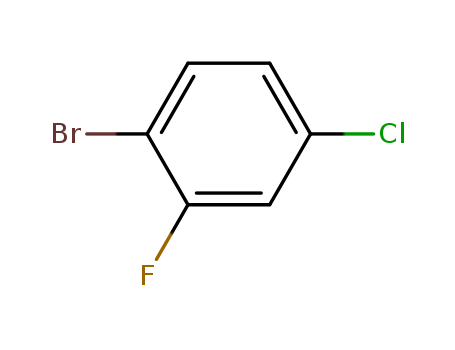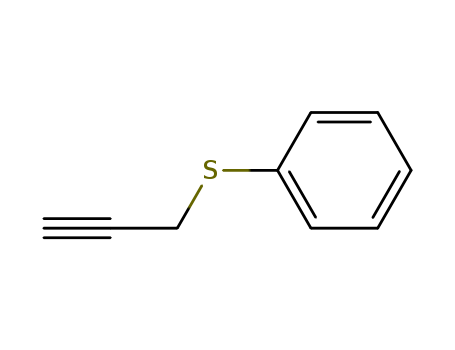
5651-88-7
- Product Name:Phenyl propargyl sulfide
- Molecular Formula:C9H8S
- Purity:99%
- Molecular Weight:148.229
Product Details;
CasNo: 5651-88-7
Molecular Formula: C9H8S
factory and supplier 5651-88-7 Phenyl propargyl sulfide in stock
- Molecular Formula:C9H8S
- Molecular Weight:148.229
- Vapor Pressure:0.24mmHg at 25°C
- Refractive Index:n20/D 1.593(lit.)
- Boiling Point:213.4 °C at 760 mmHg
- Flash Point:76.7 °C
- PSA:25.30000
- Density:1.07 g/cm3
- LogP:2.41190
PHENYL PROPARGYL SULFIDE(Cas 5651-88-7) Usage
|
General Description |
Phenyl propargyl sulfide is an organic compound with the chemical formula C9H8S. It is a colorless liquid with a garlic-like odor, derived from garlic and other Allium species. It is commonly used as a flavoring agent in the food industry and as a fragrance in cosmetics and personal care products. Phenyl propargyl sulfide also exhibits antimicrobial properties and has been studied for its potential health benefits, including anti-cancer and anti-inflammatory effects. However, |
InChI:InChI=1/C9H8S/c1-2-8-10-9-6-4-3-5-7-9/h1,3-7H,8H2
5651-88-7 Relevant articles
Discovery of a novel family of FKBP12 “reshapers” and their use as calcium modulators in skeletal muscle under nitro-oxidative stress
Aizpurua, Jesus M.,Miranda, José I.,Irastorza, Aitziber,Torres, Endika,Eceiza, Maite,Sagartzazu-Aizpurua, Maialen,Ferrón, Pablo,Aldanondo, Garazi,Lasa-Fernández, Haizpea,Marco-Moreno, Pablo,Dadie, Naroa,López de Munain, Adolfo,Vallejo-Illarramendi, Ainara
, (2021/01/25)
The hypothesis of rescuing FKBP12/RyR1 i...
Synthesis, characterization and antibacterial activity of the thioether linked 1,2,3-triazoles
Kaushik, Chander P.,Sangwan, Jyoti
supporting information, p. 3403 - 3415 (2021/09/13)
A green synthetic approach for the exped...
Direct Decarboxylative Allylation and Arylation of Aliphatic Carboxylic Acids Using Flavin-Mediated Photoredox Catalysis
Ramirez, Nieves P.,Lana-Villarreal, Teresa,Gonzalez-Gomez, Jose C.
supporting information, p. 1539 - 1550 (2019/08/07)
We describe herein a direct decarboxylat...
Photochemical Doyle-Kirmse Reaction: A Route to Allenes
Or?owska, Katarzyna,Rybicka-Jasińska, Katarzyna,Krajewski, Piotr,Gryko, Dorota
supporting information, p. 1018 - 1021 (2020/01/31)
This Letter describes the metal-free, bl...
5651-88-7 Process route
-

-
108-98-5
thiophenol

-
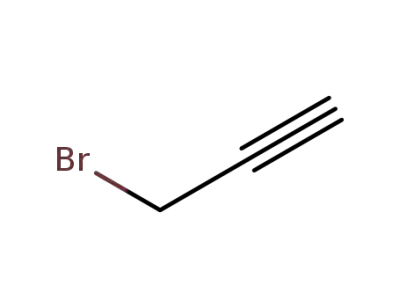
-
106-96-7
propargyl bromide

-
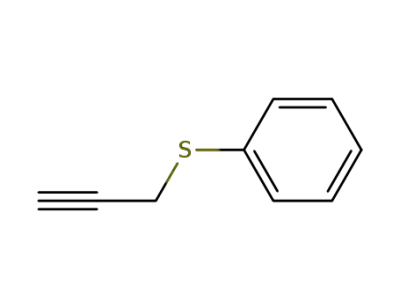
-
5651-88-7
phenyl propargyl sulfide
| Conditions | Yield |
|---|---|
|
With
sodium hydroxide; tetrabutyl ammonium fluoride;
In
benzene;
a) 0 deg C, 30 min, b) r.t., 2h;
|
100% |
|
With
triethylamine;
In
diethyl ether;
at 20 ℃;
for 2h;
|
100% |
|
With
potassium carbonate;
In
N,N-dimethyl-formamide;
at 20 ℃;
for 2h;
|
96% |
|
With
potassium carbonate;
In
N,N-dimethyl-formamide;
at 20 ℃;
for 2h;
Inert atmosphere;
|
96% |
|
With
sodium hydroxide; adogen 464;
In
water; toluene; benzene;
for 0.333333h;
|
90% |
|
With
sodium hydroxide; tetrabutylammomium bromide;
In
water; toluene; benzene;
at 20 ℃;
for 2.5h;
|
90% |
|
With
sodium ethanolate;
In
ethanol;
for 5.5h;
Ambient temperature;
|
88% |
|
thiophenol;
With
potassium carbonate;
In
N,N-dimethyl-formamide;
at 20 ℃;
for 0.5h;
Inert atmosphere;
propargyl bromide;
In
N,N-dimethyl-formamide;
at 20 ℃;
for 10h;
Inert atmosphere;
|
85% |
|
With
potassium carbonate;
In
acetone;
at 50 ℃;
|
83% |
|
Ce(72percent)NaY;
In
hexane;
for 10h;
Heating;
|
79% |
|
With
18-crown-6 ether; potassium carbonate;
In
toluene;
at 20 ℃;
for 6h;
|
78% |
|
With
sodium hydroxide; adogen 464;
In
toluene; benzene;
at 0 ℃;
for 0.5h;
|
76% |
|
With
potassium carbonate;
In
N,N-dimethyl-formamide;
at 60 ℃;
for 16h;
|
70% |
|
With
potassium carbonate;
In
N,N-dimethyl-formamide;
at 20 ℃;
|
67% |
|
thiophenol;
With
potassium carbonate;
In
acetonitrile;
at 20 ℃;
for 0.0833333h;
propargyl bromide;
In
toluene; acetonitrile;
at 50 ℃;
|
56% |
|
With
sodium ethanolate;
|
|
|
|
|
|
With
1,8-diazabicyclo[5.4.0]undec-7-ene;
In
tetrahydrofuran;
at -10 ℃;
|
|
|
With
potassium hydroxide;
In
methanol;
for 12h;
Yield given;
Ambient temperature;
|
|
|
With
potassium carbonate;
In
acetone;
for 15h;
Heating;
|
|
|
With
potassium carbonate;
In
acetone;
for 16h;
Heating;
|
|
|
With
potassium carbonate;
In
methanol;
at 0 ℃;
for 0.5h;
|
|
|
With
potassium carbonate;
In
acetone;
Heating;
|
|
|
With
potassium carbonate;
In
toluene;
Heating;
|
|
|
thiophenol;
With
sodium hydride;
In
tetrahydrofuran;
at 0 - 20 ℃;
for 0.833333h;
Inert atmosphere;
propargyl bromide;
In
tetrahydrofuran;
at 0 - 20 ℃;
for 2h;
Inert atmosphere;
|
|
|
With
potassium carbonate;
In
N,N-dimethyl-formamide;
at 10 - 25 ℃;
|
|
|
With
potassium carbonate;
In
ethanol;
at 20 ℃;
for 12h;
|
|
|
With
potassium carbonate;
In
N,N-dimethyl-formamide;
at 10 - 25 ℃;
|
|
|
With
triethylamine;
In
water;
at 20 ℃;
for 2h;
|
|
|
With
triethylamine;
In
dichloromethane; toluene;
at 20 ℃;
for 2h;
Cooling with ice;
|
|
|
With
triethylamine;
In
diethyl ether;
at 25 ℃;
for 2h;
|
|
|
With
potassium carbonate;
In
N,N-dimethyl-formamide;
|
|
|
With
potassium carbonate;
In
acetone;
for 24h;
Reflux;
Inert atmosphere;
|
|
|
With
potassium carbonate;
In
N,N-dimethyl-d6-formamide;
at 10 - 25 ℃;
|
-
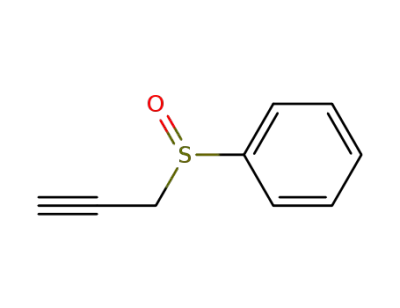
-
13865-11-7
phenyl propargyl sulfoxide

-
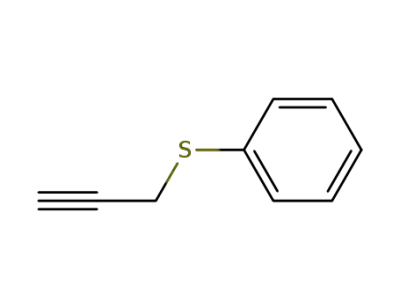
-
5651-88-7
phenyl propargyl sulfide
| Conditions | Yield |
|---|---|
|
With
triphenyl phosphite;
[MoO2Cl2(dmf)2];
In
acetonitrile;
Heating;
|
82% |
|
With
1,2,3-trimethoxybenzene; oxalyl dichloride;
In
dichloromethane;
at 0 ℃;
for 0.00166667h;
chemoselective reaction;
Inert atmosphere;
|
81% |
|
With
iododioxobis(triphenylphosphine)rhenium(V); phenylsilane;
In
tetrahydrofuran;
for 0.666667h;
chemoselective reaction;
Reflux;
|
80% |
|
With
ReOBr2(hmpbta)(PPh3); phenylsilane;
In
tetrahydrofuran;
for 1.25h;
Reagent/catalyst;
Time;
chemoselective reaction;
Reflux;
|
76% |
|
With
trimethylphenylsilane; ReOBr2(2-(2-hydroxy-5-methylphenyl)benzotriazole-(H))(PPh3);
In
tetrahydrofuran;
for 1.25h;
Reagent/catalyst;
chemoselective reaction;
Reflux;
|
76% |
|
With
per-rhenic acid; benzo[1,3,2]dioxaborole;
In
tetrahydrofuran;
at 20 ℃;
for 0.0833333h;
chemoselective reaction;
|
66% |
|
With
Dimethylphenylsilane;
In
1,4-dioxane;
at 30 ℃;
for 6h;
Inert atmosphere;
|
89 %Chromat. |
5651-88-7 Upstream products
-
930-69-8
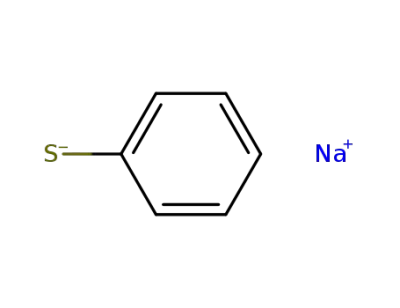
sodium thiophenolate
-
106-96-7

propargyl bromide
-
6165-75-9
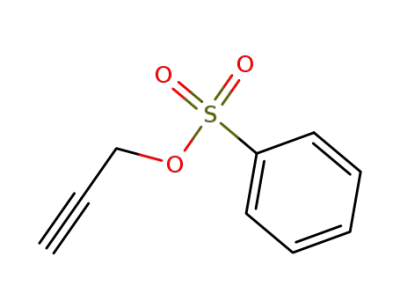
propargyl benzenesulfonate
-
108-98-5

thiophenol
5651-88-7 Downstream products
-
13864-98-7
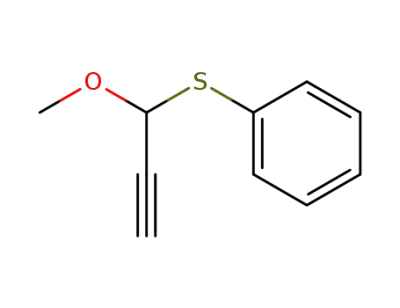
1-Methoxy-1-phenylthio-2-propin
-
71570-22-4
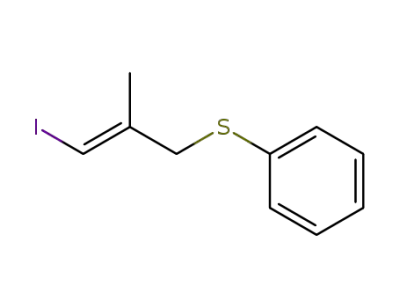
(E)-1-Iodo-2-methyl-3-(thiophenoxy)-1-propene
-
624-67-9
Propargylic aldehyde
-
6212-77-7
1-phenylthio-1-propyne
Relevant Products
-
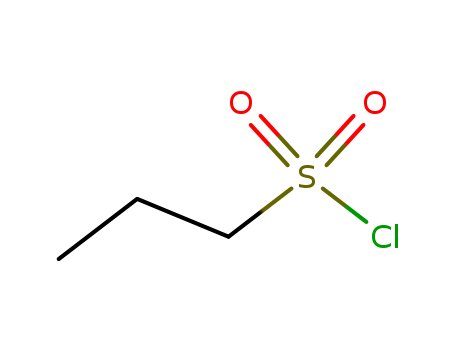
Propanesulphonyl chloride
CAS:10147-36-1
-
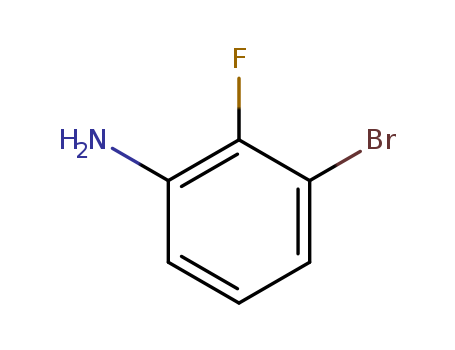
3-BroMo-2-fluoroaniline
CAS:58534-95-5
-
1-Bromo-4-chloro-2-fluorobenzene
CAS:1996-29-8