4805-22-5
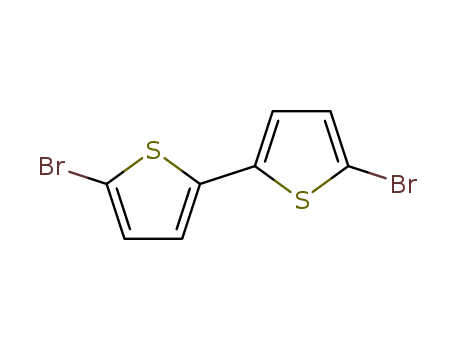
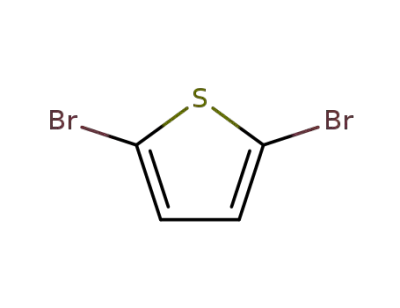
- Product Name:5,5'-Dibromo-2,2'-bithiophene
- Molecular Formula:C8H4Br2S2
- Purity:99%
- Molecular Weight:324.06
Product Details;
CasNo: 4805-22-5
Molecular Formula: C8H4Br2S2
Appearance: white to light yellow shiny flakes
factory and supplier 4805-22-5 5,5'-Dibromo-2,2'-bithiophene in stock
- Molecular Formula:C8H4Br2S2
- Molecular Weight:324.06
- Appearance/Colour:white to light yellow shiny flakes
- Melting Point:144-146 °C(lit.)
- Boiling Point:318.6 °C at 760 mmHg
- Flash Point:146.5 °C
- PSA:56.48000
- Density:1.951 g/cm3
- LogP:5.00160
4805-22-5 Relevant articles
Emission enhancement of a carbazole-based fluorophore on a quantum dot surface
Kumar De, Puran,Neckers, Douglas C.
, p. 363 - 368 (2013)
A novel carbazole-based fluorophore 5-(c...
New fused thiophene derivatives as promising building blocks for optoelectronic devices
Keshtov,Kuklin,Osipov,Topchii,Konstantinov,Gamov,Khokhlov
, p. 50 - 56 (2015)
-
Synthesis and Characterization of α,α'-Bis(aminomethyl)oligothiophenes and Their Related Compounds
Muguruma, Hitoshi,Saito, Takashi,Sasaki, Satoshi,Hotta, Shu,Karube, Isao
, p. 173 - 178 (1996)
We have synthesized and characterized a ...
A star-shaped oligothiophene with triphenylamine as core and octyl cyanoacetate as end groups for solution-processed organic solar cells
Shen, Suling,Gao, Lei,He, Chang,Zhang, Zhanjun,Sun, Qingjiang,Li, Yongfang
, p. 875 - 881 (2013)
A new star-shaped D-π-A molecule with tr...
Improved synthesis of a quaterthiophene-triazine-diamine derivative, a promising molecule to study pathogenic prion proteins
Rodrigues, Alysson Duarte,Imberdis, Thibaut,Perrier, Véronique,Robitzer, Mike
, p. 368 - 373 (2015)
The 6,6′-([2,2′:5′,2″:5″,2-quaterthiophe...
Synthesis of 2-Bromo-2′-phenyl-5,5′-thiophene: Suzuki reaction versus Negishi reaction
Wang, Nai-Xing
, p. 2119 - 2124 (2003)
2-Bromo-2′-phenyl-5,5′-thiophene was syn...
Synthesis and structure of tetrathiophene with a chiral 1,1′-binaphthyl kink
Rajca,Wang,Pawitranon,Brett,Stezowski
, p. 1060 - 1061 (2001)
A chiral oligothiophene, possessing in-c...
Aryl halide and synthesis method and application thereof
-
Paragraph 0118-0120, (2020/06/02)
The invention discloses a synthesis meth...
Visible-light-promoted oxidative halogenation of (hetero)arenes
Jiang, Xuefeng,Li, Yiming,Lu, Lingling
supporting information, p. 5989 - 5994 (2020/10/18)
Organic halides are critical building bl...
Pd-Catalyzed Aerobic Oxidative Coupling of Thiophenes: Synergistic Benefits of Phenanthroline Dione and a Cu Cocatalyst
Tereniak, Stephen J.,Bruns, David L.,Stahl, Shannon S.
supporting information, p. 20318 - 20323 (2020/12/01)
Substituted bithiophenes are prominent f...
Synthesis and characterization of new D-π-A and A-π-D-π-A type oligothiophene derivatives
Pandolfi, Fabiana,Rocco, Daniele,Mattiello, Leonardo
supporting information, p. 3018 - 3025 (2019/03/21)
In this work, we present a series of new...
4805-22-5 Process route
-
-
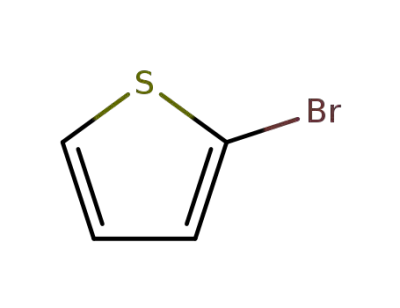
1003-09-4
2-bromothiophene

-
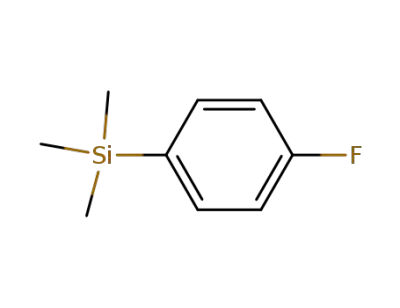
-
455-17-4
(p-fluorophenyl)trimethylsilane

-
-
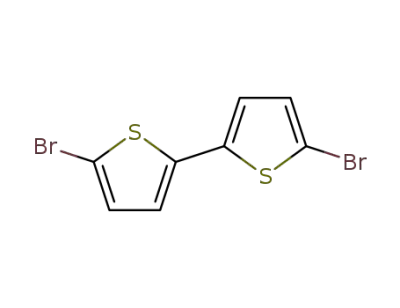
4805-22-5
5,5'-dibromo-2,2'-bisthiophene

-
-
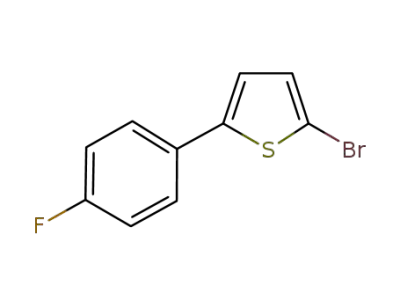
1073313-97-9
2-bromo-5-(4-fluorophenyl)thiophene

-
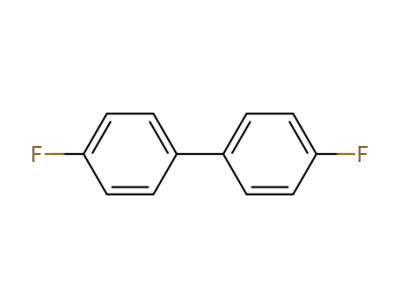
-
398-23-2
4,4'-difluorobiphenyl
| Conditions | Yield |
|---|---|
|
With
gold(lll) acetate; (1S)-10-camphorsulfonic acid;
In
chloroform-d1; d(4)-methanol;
at 25 ℃;
for 2h;
|
-
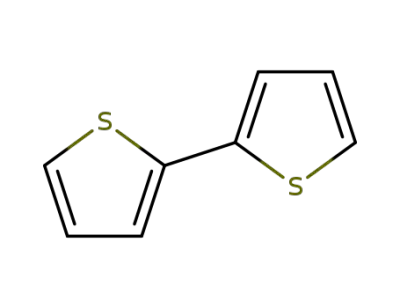
-
492-97-7
2,2'-Bithiophene

-

-
4805-22-5
5,5'-dibromo-2,2'-bisthiophene
| Conditions | Yield |
|---|---|
|
With
mono(N,N,N-trimethylbenzenaminium) tribromide;
zinc(II) chloride;
In
acetic acid;
for 27h;
Ambient temperature;
|
99% |
|
With
N-Bromosuccinimide; acetic anhydride;
|
99% |
|
With
N-Bromosuccinimide; acetic acid;
In
chloroform;
at 0 ℃;
for 0.5h;
Inert atmosphere;
|
99.8% |
|
With
N-Bromosuccinimide;
In
dichloromethane;
at 20 ℃;
for 0.05h;
Ultrasonic irradiation;
|
98% |
|
With
N-Bromosuccinimide; acetic acid;
In
chloroform;
at 70 ℃;
for 4h;
|
98% |
|
With
N-Bromosuccinimide;
In
chloroform; acetic acid;
at 70 ℃;
for 4h;
|
95.1% |
|
With
2-bromo-3,4-dichloropyridazin-3(2H)-one; zinc dibromide;
In
dichloromethane;
at 20 ℃;
for 0.0833333h;
regioselective reaction;
|
94% |
|
With
N-Bromosuccinimide;
In
N,N-dimethyl-formamide;
for 8h;
|
93% |
|
With
N-Bromosuccinimide;
In
chloroform; acetic acid;
for 6h;
Reflux;
Inert atmosphere;
|
93% |
|
With
N-Bromosuccinimide;
In
N,N-dimethyl-formamide;
for 2h;
Reflux;
|
93% |
|
With
N-Bromosuccinimide;
In
N,N-dimethyl-formamide;
at 0 - 20 ℃;
Inert atmosphere;
|
92% |
|
With
bromine; acetic acid;
at 30 ℃;
for 2h;
|
90% |
|
With
N-Bromosuccinimide; acetic acid;
at 20 ℃;
for 3h;
|
90% |
|
With
N-Bromosuccinimide;
In
N,N-dimethyl-formamide;
Cooling with ice;
|
90% |
|
With
N-Bromosuccinimide;
In
acetone;
at 20 - 60 ℃;
|
90% |
|
With
N-Bromosuccinimide;
In
N,N-dimethyl-formamide;
at 20 ℃;
for 3h;
|
88% |
|
With
N-Bromosuccinimide;
In
N,N-dimethyl-formamide;
at 3 - 8 ℃;
|
87.5% |
|
With
N-Bromosuccinimide;
In
N,N-dimethyl-formamide;
at 20 ℃;
|
85% |
|
With
N-Bromosuccinimide;
|
85% |
|
With
N-Bromosuccinimide;
In
N,N-dimethyl-formamide;
at 20 ℃;
for 3h;
|
85.69% |
|
With
N-Bromosuccinimide;
In
N,N-dimethyl-formamide;
|
84% |
|
With
N-Bromosuccinimide;
In
N,N-dimethyl-formamide;
at -20 ℃;
for 4h;
|
80% |
|
With
bromine; sodium hydrogencarbonate;
In
chloroform;
for 1h;
Ambient temperature;
|
77% |
|
With
dihydrogen peroxide; ammonium bromide; acetic acid;
In
water;
for 16h;
Time;
Solvent;
Reagent/catalyst;
regioselective reaction;
Green chemistry;
|
76% |
|
With
n-butyllithium; bromine;
In
tetrahydrofuran;
at -78 ℃;
|
60% |
|
With
water; sodium bisulfate hydrate; sodium bromide;
In
acetonitrile;
for 96h;
Irradiation;
|
57% |
|
With
sodium hydrogensulfate monohydrate; water; sodium bromide;
In
acetonitrile;
at 20 ℃;
for 96h;
Schlenk technique;
Irradiation;
Green chemistry;
|
57% |
|
With
N-Bromosuccinimide;
In
N,N-dimethyl-formamide;
at 0 ℃;
for 12h;
|
51% |
|
With
lead(IV) tetraacetate; lithium bromide;
In
chloroform;
at 20 ℃;
for 4h;
regioselective reaction;
|
28% |
|
With
N-Bromosuccinimide;
In
acetic acid;
|
|
|
With
N-Bromosuccinimide;
In
methanol;
|
|
|
With
N-Bromosuccinimide;
In
N,N-dimethyl-formamide;
|
|
|
With
N-Bromosuccinimide;
In
N,N-dimethyl-formamide;
|
|
|
With
N-Bromosuccinimide;
In
N,N-dimethyl-formamide;
|
|
|
With
N-Bromosuccinimide;
|
4805-22-5 Upstream products
-
3141-27-3
2,5-dibromothiophen
-
492-97-7

2,2'-Bithiophene
-
19162-80-2
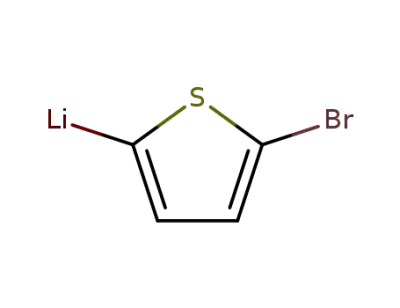
5-bromo-[2]thienyl lithium
-
1003-09-4

2-bromothiophene
4805-22-5 Downstream products
-
88493-55-4
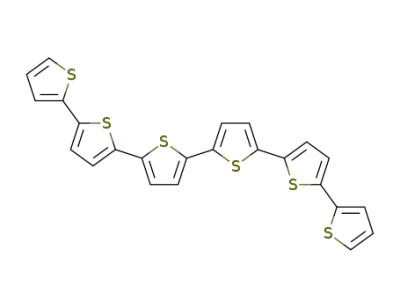
sexithiophene
-
153561-79-6
5,5'''-didodecyl-2,2':5',2'':5'',2'''-quaterthiophene
-
125143-53-5
3,3',5,5'-tetrabromo-2,2'-bithiophene
-
162151-09-9

3,3?-didodecyl-2,2′;5′,2″;5″,2?-quaterthiophene
Relevant Products
-
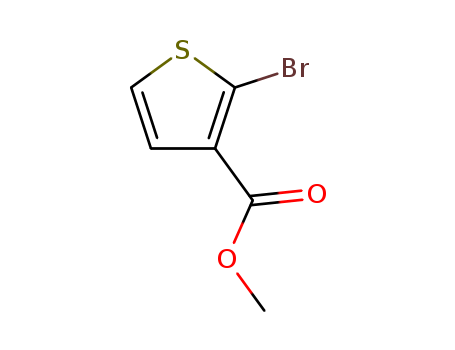
Methyl 2-bromothiophene-3-carboxylate
CAS:76360-43-5
-
Dibromobis(triphenylphosphine)nickel(II)
CAS:14126-37-5
-
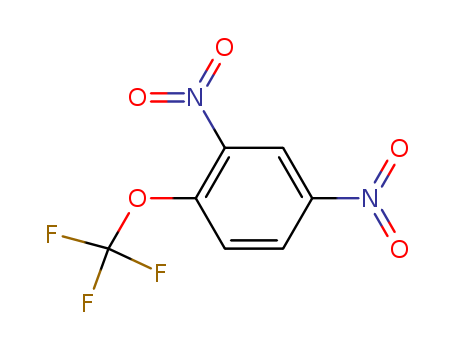
2,4-Dinitro-1-(trifluoromethoxy)benzene
CAS:655-07-2