14126-37-5
- Product Name:Dibromobis(triphenylphosphine)nickel(II)
- Molecular Formula:C36H30Br2NiP2
- Purity:99%
- Molecular Weight:743.08
Product Details;
CasNo: 14126-37-5
Molecular Formula: C36H30Br2NiP2
Appearance: dark green crystalline powder or crystals
factory and supplier 14126-37-5 Dibromobis(triphenylphosphine)nickel(II) in stock
- Molecular Formula:C36H30Br2NiP2
- Molecular Weight:743.08
- Appearance/Colour:dark green crystalline powder or crystals
- Vapor Pressure:4.74E-05mmHg at 25°C
- Melting Point:219-223 °C(lit.)
- Boiling Point:360 °C at 760 mmHg
- Flash Point:181.7 °C
- PSA:27.18000
- LogP:8.58080
BIS(TRIPHENYLPHOSPHINE)NICKEL(II) BROMIDE(Cas 14126-37-5) Usage
InChI:InChI=1/2C18H15P.2BrH.Ni/c2*1-4-10-16(11-5-1)19(17-12-6-2-7-13-17)18-14-8-3-9-15-18;;;/h2*1-15H;2*1H;/q;;;;+2/p-2
14126-37-5 Relevant articles
Catalysis of Cross-Coupling and Homocoupling Reactions of Aryl Halides Utilizing Ni(0), Ni(I), and Ni(II) Precursors; Ni(0) Compounds as the Probable Catalytic Species but Ni(I) Compounds as Intermediates and Products
Manzoor, Adeela,Wienefeld, Patrick,Baird, Michael C.,Budzelaar, Peter H.M.
, p. 3508 - 3519 (2017/10/03)
Both Ni(0) and Ni(I) compounds are belie...
Specific features of nickel-catalyzed synthesis of poly(butyl (meth)acrylates)
Grishin,Valetova,Il'Ichev
, p. 1965 - 1969 (2012/03/12)
The activity of the catalytic system NiB...
LINEAR PYRIDAZINE AND PYRROLE COMPOUNDS, METHOD FOR OBTAINING THEM AND APPLICATIONS
-
Page/Page column 32, (2010/02/16)
The present invention relates to linear ...
Synthesis of 2-(N-arylimino-κN-methyl)pyrrolide-κN complexes of nickel
Pérez-Puente, Pilar,de Jesús, Ernesto,Flores, Juan C.,Gómez-Sal, Pilar
, p. 3902 - 3906 (2009/04/06)
2-(N-aryliminomethyl)pyrrole precursors ...
14126-37-5 Process route
-
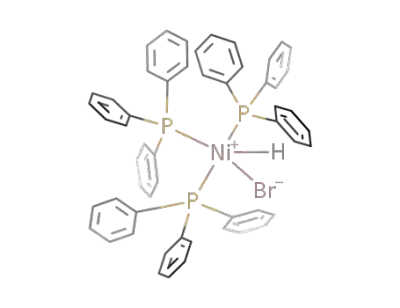
-
57584-08-4
((C6H5)3P)3Ni(H)Br

-
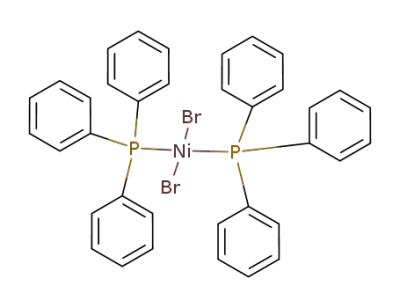
-
36673-36-6,111408-20-9,14126-37-5,54053-52-0
dibromobis(triphenylphosphine)nickel(II)

-
-
13007-90-4
bis(triphenylphosphine)nickel(0) dicarbonyl
| Conditions | Yield |
|---|---|
|
With
carbon monoxide;
In
tetrahydrofuran;
byproducts: (C6H5)3P; (Ar), to nickel complex added THF previously saturated with CO, CO passed in for 2.5 h at -20°C, pptd.; ppt. after washing (THF, pentane) obtained (Ph3P)2NiBr2, filtrate evaptd., washed (pentane) extracted (ether), chromy (Al2O3, benzene/petroleumether 1:1), elem. anal., IR;
|
-
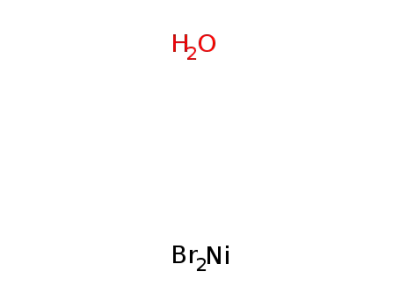
-
nickel(II) bromide monohydrate

-
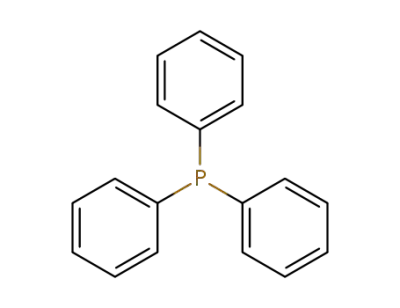
-
603-35-0
triphenylphosphine

-

-
36673-36-6,111408-20-9,14126-37-5,54053-52-0
dibromobis(triphenylphosphine)nickel(II)
| Conditions | Yield |
|---|---|
|
In
butan-1-ol;
at 20 ℃;
Reflux;
|
60% |
14126-37-5 Upstream products
-
57584-08-4

((C6H5)3P)3Ni(H)Br
-
31200-71-2

Ni(acetylacetonate)2(triphenylphosphine)
-
760-19-0
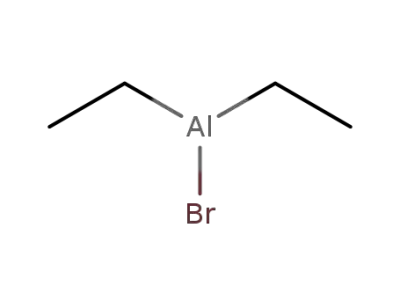
diethylaluminum bromide
-
74-96-4
ethyl bromide
14126-37-5 Downstream products
-
7440-02-0

nickel
-
542-52-9
Dibutyl carbonate
-
2050-60-4
di-n-butyl oxalate
-
13463-39-3
tetracarbonyl nickel
Relevant Products
-
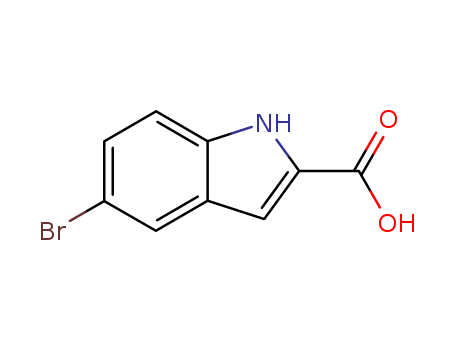
5-Bromoindole-2-carboxylic acid
CAS:7254-19-5
-
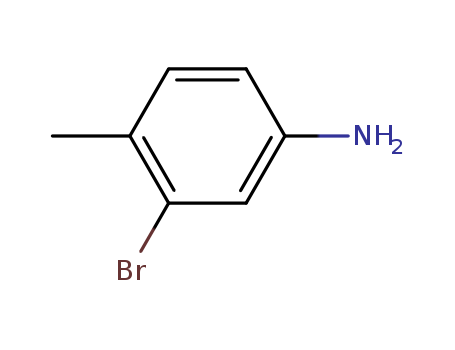
3-Bromo-4-methylaniline
CAS:7745-91-7
-
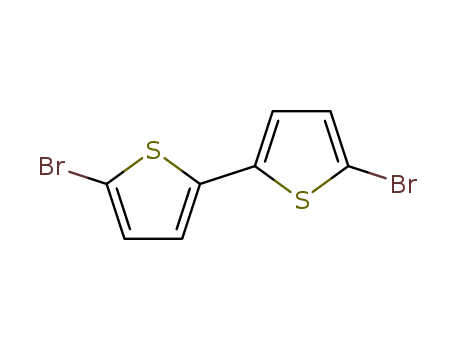
5,5'-Dibromo-2,2'-bithiophene
CAS:4805-22-5