576-26-1
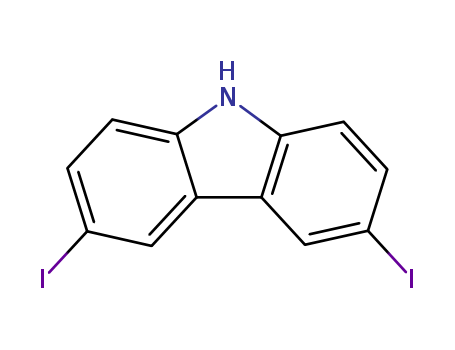
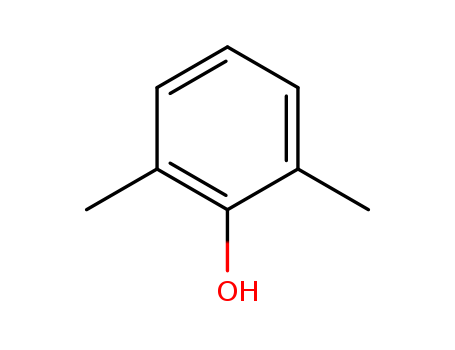
- Product Name:2,6-Dimethylphenol
- Molecular Formula:C8H10O
- Purity:99%
- Molecular Weight:122.167
Product Details;
CasNo: 576-26-1
Molecular Formula: C8H10O
Appearance: Colorless to off-white crystalline olid
factory and supplier 576-26-1 2,6-Dimethylphenol in stock
- Molecular Formula:C8H10O
- Molecular Weight:122.167
- Appearance/Colour:Colorless to off-white crystalline olid
- Vapor Pressure:0.2 hPa (20 °C)
- Melting Point:43-45 °C(lit.)
- Refractive Index:1.5371
- Boiling Point:201.1 °C at 760 mmHg
- PKA:pK1:10.59 (25°C)
- Flash Point:78.3 °C
- PSA:20.23000
- Density:1.15 g/cm3
- LogP:2.00900
2,6-Dimethylphenol(Cas 576-26-1) Usage
|
Preparation |
From coal tar oil or coal hydrogenation.Synthesis of 2,6-dimethylphenol: The gas-phase catalytic reaction of phenol and methanol is carried out, and then purified by rectification, and the product purity can reach more than 99%.Selective phenol methylation to 2,6-dimethylphenol in a fluidized bed of iron-chromium mixed oxide catalyst with o–cresol circulationCatalytic synthesis of 2,6-dimethylphenol from methanol and cyclohexanone over titanium oxide-supported vanadium oxide catalysts |
|
Reactivity Profile |
2,6-Dimethylphenol is incompatible with bases, acid chlorides, acid anhydrides, and oxidizing agents. Corrodes steel, brass, copper, and copper alloys. |
|
Fire Hazard |
2,6-Dimethylphenol is combustible. |
|
Flammability and Explosibility |
Nonflammable |
|
Purification Methods |
Fractionally distil 2,6-xylenol under nitrogen, crystallise it from *benzene or hexane, and sublime it at 38o/10mm. [Beilstein 6 IV 3122.] |
|
General Description |
2,6-Dimethylphenol (2,6-xylenol) is a phenol derivative that serves as a key substrate in synthetic chemistry, particularly in studies involving thiomethylation and transition-metal-mediated C–H bond functionalization. It demonstrates reactivity in ortho-substitution reactions, as evidenced by its participation in thiomethylation processes using alkyl diethylaminomethyl sulfides, yielding mono- and disubstituted products selectively. Additionally, its sp3 C–H bonds can be cleaved stoichiometrically by ruthenium complexes, enabling transformations into allylic phenols and benzopyrans, highlighting its utility in metal-catalyzed organic synthesis. |
|
Application |
2,6-dimethylphenol is generally applied in industry as a monomer in polymerization reaction. For the production of polyphenylene ether resins, polyester and polyether resins. |
|
Aroma threshold values |
Detection: 400 ppb |
InChI:InChI=1/C8H10O/c1-6-4-3-5-7(2)8(6)9/h3-5,9H,1-2H3
576-26-1 Relevant articles
Impact of oxygen vacancies in Ni supported mixed oxide catalysts on anisole hydrodeoxygenation
Ali, Hadi,Kansal, Sushil Kumar,Lauwaert, Jeroen,Saravanamurugan, Shunmugavel,Thybaut, Joris W.,Vandevyvere, Tom
, (2022/03/02)
The hydrodeoxygenation (HDO) activity of...
Catalytic Activation of Unstrained C(Aryl)-C(Alkyl) Bonds in 2,2′-Methylenediphenols
Dong, Guangbin,Ratchford, Benjamin L.,Xue, Yibin,Zhang, Rui,Zhu, Jun
, p. 3242 - 3249 (2022/02/23)
Catalytic activation of unstrained and n...
A mild and practical method for deprotection of aryl methyl/benzyl/allyl ethers with HPPh2andtBuOK
Pan, Wenjing,Li, Chenchen,Zhu, Haoyin,Li, Fangfang,Li, Tao,Zhao, Wanxiang
, p. 7633 - 7640 (2021/09/22)
A general method for the demethylation, ...
Catalyst-free rapid conversion of arylboronic acids to phenols under green condition
Dong, Zhenhua,Liu, Mengmeng,Pan, Hongguo
, (2021/09/06)
A catalyst-free and solvent-free method ...
576-26-1 Process route
-
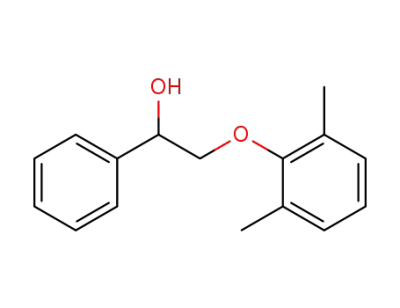
-
2-(2,6-dimethylphenoxy)-1-phenylethanol

-
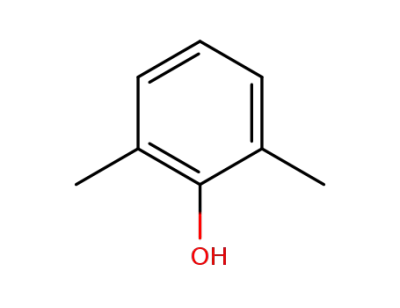
-
576-26-1,25134-01-4
2.6-dimethylphenol

-
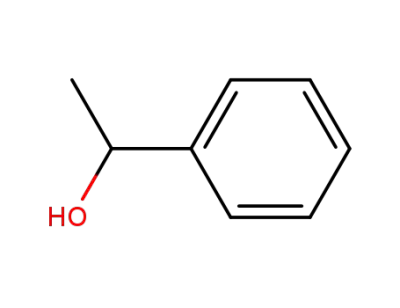
-
98-85-1,13323-81-4
1-Phenylethanol

-
-
C16H18O
| Conditions | Yield |
|---|---|
|
With
PdNi7; hydrogen;
at 130 ℃;
for 16h;
under 760.051 Torr;
Ionic liquid;
|
87 %Chromat. 87 %Chromat. 12 %Chromat. |
-
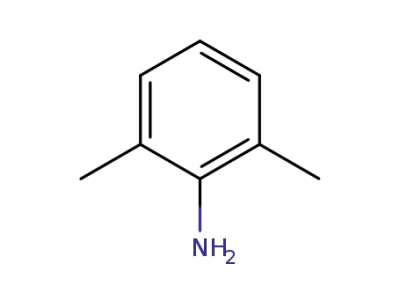
-
87-62-7
2,6-dimethylaniline

-

-
576-26-1,25134-01-4
2.6-dimethylphenol

-
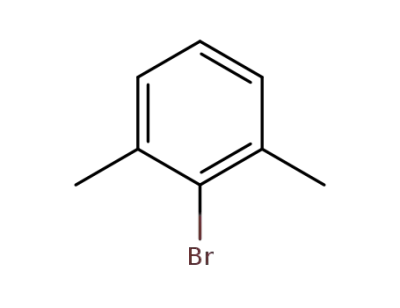
-
576-22-7
2-Bromo-m-xylene
| Conditions | Yield |
|---|---|
|
With
sulfuric acid; sodium nitrite;
Diazotization.Kochen der erhaltenen Diazoniumsalz-Loesung mit CuBr;
|
576-26-1 Upstream products
-
2816-57-1

2,6-dimethylcyclohexanone
-
824-42-0
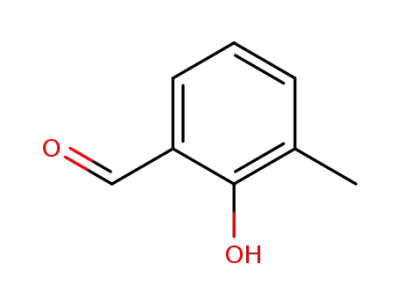
2-methoxy-3-methylbenzaldehyde
-
23802-11-1
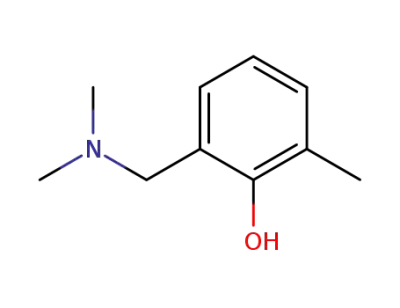
2-(dimethylamino-methyl)-6-methyl-phenol
-
111-87-5
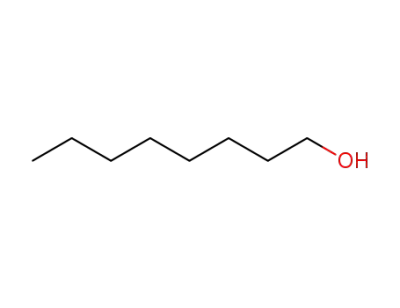
octanol
576-26-1 Downstream products
-
42900-97-0
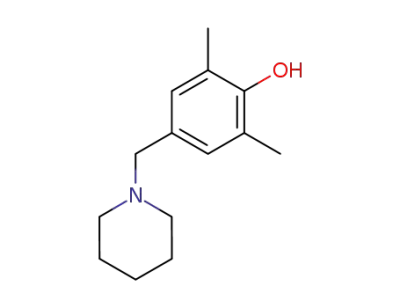
1-<(4-Hydroxy-3,5-dimethyl-phenyl)-methyl>-piperidin
-
112896-07-8
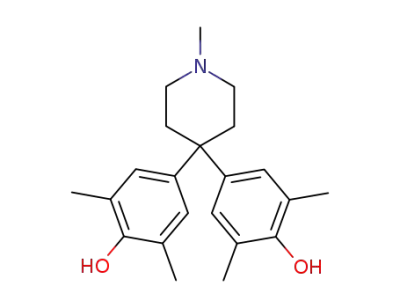
4,4-bis-(4-hydroxy-3,5-dimethyl-phenyl)-1-methyl-piperidine
-
29237-05-6
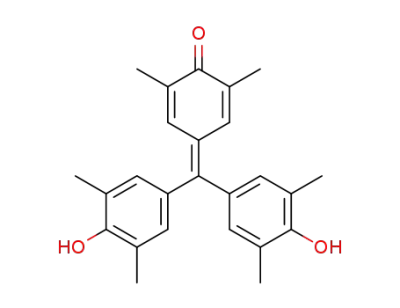
4-(4,4'-dihydroxy-3,5,3',5'-tetramethyl-benzhydrylidene)-2,6-dimethyl-cyclohexa-2,5-dienone
-
3689-45-0
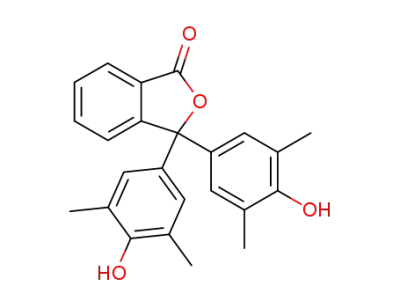
3,3-bis(4-hydroxy-3,5-dimethylphenyl)isobenzofuran-1(3H)-one
Relevant Products
-
DIBASIC ESTER
CAS:95481-62-2
-
3,6-Diiodo-9H-carbazole
CAS:57103-02-3
-
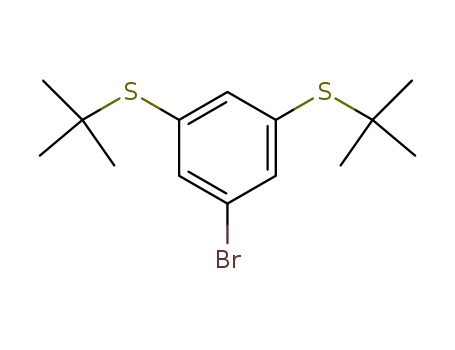
1-Bromo-3,5-bis(tert-butylthio)benzene
CAS:795274-44-1