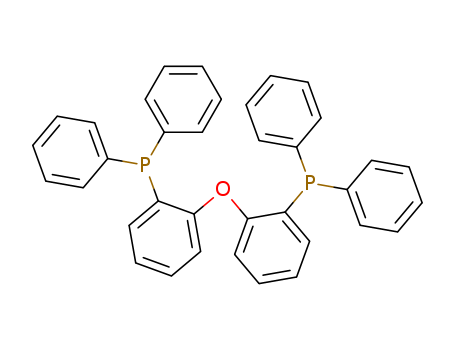
166330-10-5
- Product Name:Bis(2-diphenylphosphinophenyl)ether
- Molecular Formula:C36H28OP2
- Purity:99%
- Molecular Weight:538.565
Product Details;
CasNo: 166330-10-5
Molecular Formula: C36H28OP2
Appearance: White to off-white crystalline powder or crystals
factory and supplier 166330-10-5 Bis(2-diphenylphosphinophenyl)ether in stock
- Molecular Formula:C36H28OP2
- Molecular Weight:538.565
- Appearance/Colour:White to off-white crystalline powder or crystals
- Vapor Pressure:0mmHg at 25°C
- Melting Point:184-187 °C(lit.)
- Boiling Point:608.19 °C at 760 mmHg
- Flash Point:404.905 °C
- PSA:36.41000
- LogP:6.99530
(OXYDI-2,1-PHENYLENE)BIS(DIPHENYLPHOSPHINE)(Cas 166330-10-5) Usage
|
Reaction |
Useful as a ligand in the Pd-catalyzed formation of diaryl amines. Has been recently applied to the C3 benzylation of indoles. Has been recently applied to the monoallylation of ammonia. Ligand used in the palladium-catalyzed, aerobic oxidation coupling of acyl chlorides with arylboronic acids. Ligand used in carbonylation of aryl iodides. Ligand used in the direct C-H arylation of benzothiodiazoles. Ligand used in stereo-retentive azacyclization of propargylic carbonates. Ligand used in palladium catalyzed benzyne trimerization. |
|
General Description |
This product has been enhanced for catalytic efficiency. |
InChI:InChI=1/C36H28OP2/c1-5-17-29(18-6-1)38(30-19-7-2-8-20-30)35-27-15-13-25-33(35)37-34-26-14-16-28-36(34)39(31-21-9-3-10-22-31)32-23-11-4-12-24-32/h1-28H
166330-10-5 Relevant articles
Early transition metal compound and preparation method and intermediate thereof and application of early transition metal compound in polymerization of olefin
-
Paragraph 0294; 0296; 0298; 0299, (2019/11/13)
The invention relates to the field of ca...
Electrophilic Phosphonium Cation-Mediated Phosphane Oxide Reduction Using Oxalyl Chloride and Hydrogen
Stepen, Arne J.,Bursch, Markus,Grimme, Stefan,Stephan, Douglas W.,Paradies, Jan
supporting information, p. 15253 - 15256 (2018/10/24)
The metal-free reduction of phosphane ox...
Convergent modulation of singlet and triplet excited states of phosphine-oxide hosts through the management of molecular structure and functional-group linkages for low-voltage-driven electrophosphorescence
Han, Chunmiao,Zhang, Zhensong,Xu, Hui,Xie, Guohua,Li, Jing,Zhao, Yi,Deng, Zhaopeng,Liu, Shiyong,Yan, Pengfei
, p. 141 - 154 (2013/03/13)
The controllable tuning of the excited s...
Allyl acetate hydroformylation process
-
Page/Page column 3, (2011/06/19)
A process for the production of 4-acetox...
166330-10-5 Process route
-
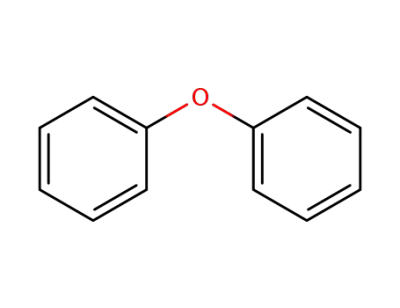
-
101-84-8
diphenylether

-
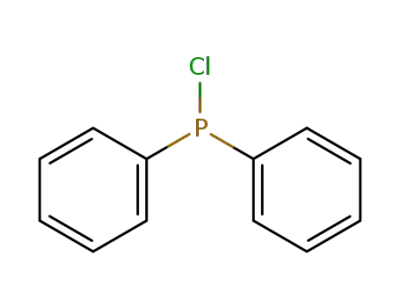
-
1079-66-9,74391-44-9
chloro-diphenylphosphine

-
![bis[2-(diphenylphosphino)phenyl] ether](/upload/2026/1/eeeea310-19f3-43a9-8d86-db3978e9e48c.png)
-
166330-10-5
bis[2-(diphenylphosphino)phenyl] ether
| Conditions | Yield |
|---|---|
|
diphenylether;
With
n-butyllithium;
In
hexane;
at -78 - 25 ℃;
for 17h;
chloro-diphenylphosphine;
In
hexane;
at 20 ℃;
for 16h;
Cooling with ice;
|
87% |
|
diphenylether;
With
n-butyllithium; N,N,N,N,-tetramethylethylenediamine;
In
tetrahydrofuran; hexane;
at 20 ℃;
for 16h;
chloro-diphenylphosphine;
In
tetrahydrofuran; hexane; Petroleum ether;
at 20 ℃;
for 24h;
|
78% |
|
With
n-butyllithium;
|
58% |
|
diphenylether;
With
N,N,N,N,-tetramethylethylenediamine; sec.-butyllithium;
In
diethyl ether; hexane;
at 20 ℃;
for 16h;
Inert atmosphere;
chloro-diphenylphosphine;
In
diethyl ether; hexane;
for 16h;
Inert atmosphere;
|
-
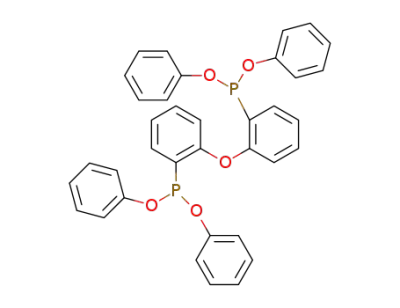
-
bis(2-diphenyloxyphosphino)phenyl ether

-
![bis[2-(diphenylphosphino)phenyl] ether](/upload/2026/1/eeeea310-19f3-43a9-8d86-db3978e9e48c.png)
-
166330-10-5
bis[2-(diphenylphosphino)phenyl] ether
| Conditions | Yield |
|---|---|
|
With
tributylphosphine; iodine;
In
tetrahydrofuran; acetonitrile;
at 20 ℃;
for 0.166667h;
Inert atmosphere;
|
94% |
166330-10-5 Upstream products
-
101-84-8

diphenylether
-
1079-66-9

chloro-diphenylphosphine
-
808142-23-6
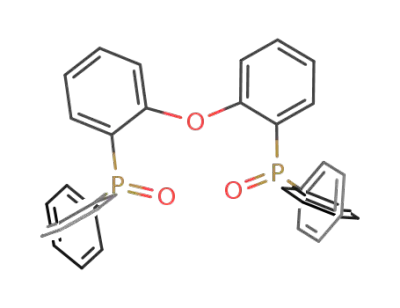
bis[2-(diphenylphosphino)phenyl] ether oxidebis[2-(diphenylphosphino)phenyl] ether oxide
166330-10-5 Downstream products
-
101-60-0
porphyrin
-
1017579-50-8
(O(C6H4P(C6H5)2)2NP(O)(OC6H5)2)
Relevant Products
-
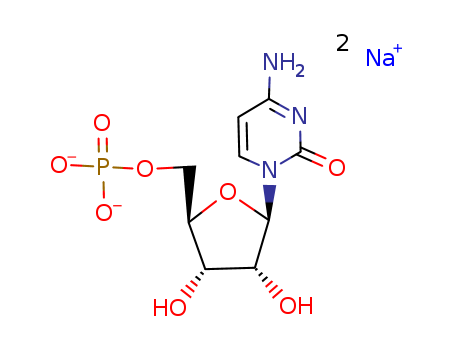
Cytidine 5'-monophosphate disodium salt
CAS:6757-06-8
-
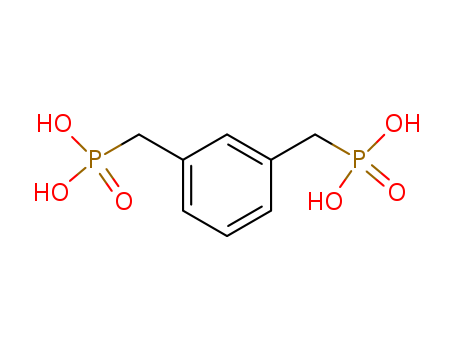
M-Xylylenediphosphonic Acid
CAS:144052-40-4
-
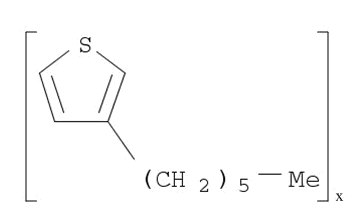
Poly(3-hexylthiophene-2,5-diyl)
CAS:104934-50-1