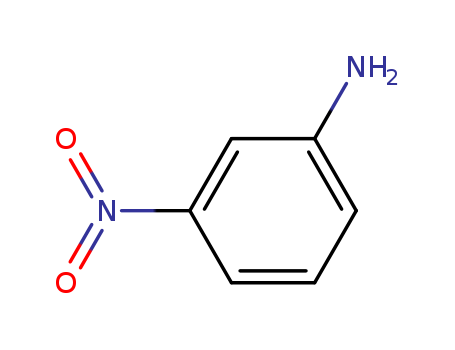
99-09-2
- Product Name:3-Nitroaniline
- Molecular Formula:C6H6N2O2
- Purity:99%
- Molecular Weight:138.126
Product Details;
CasNo: 99-09-2
Molecular Formula: C6H6N2O2
Appearance: ochre-yellow to orange crystalline powder
factory and supplier 99-09-2 3-Nitroaniline in stock
- Molecular Formula:C6H6N2O2
- Molecular Weight:138.126
- Appearance/Colour:ochre-yellow to orange crystalline powder
- Vapor Pressure:1 mm Hg ( 119 °C)
- Melting Point:111-114 °C(lit.)
- Refractive Index:1.626
- Boiling Point:307 °C at 760 mmHg
- PKA:2.466(at 25℃)
- Flash Point:139.5 °C
- PSA:71.84000
- Density:1.333 g/cm3
- LogP:2.28140
3-Nitroaniline(Cas 99-09-2) Usage
|
Preparation |
M-nitroaniline partial reduction. |
|
Synthesis Reference(s) |
The Journal of Organic Chemistry, 45, p. 4992, 1980 DOI: 10.1021/jo01312a039Tetrahedron Letters, 30, p. 251, 1989 DOI: 10.1016/S0040-4039(00)95173-6Chemical and Pharmaceutical Bulletin, 34, p. 2013, 1986 DOI: 10.1248/cpb.34.2013 |
|
Air & Water Reactions |
Insoluble in water. |
|
Reactivity Profile |
Thermal stability of 3-Nitroaniline is reduced by various impurities. 3-Nitroaniline may be sensitive to prolonged exposure to light. 3-Nitroaniline may react explosively with ethylene oxide at 266° F. 3-Nitroaniline is incompatible with acids (nitric, sulfuric), acid chlorides, acid anhydrides, chloroformates and strong oxidizing agents. . Unstable when heated. |
|
Hazard |
Moderate fire risk. Toxic when absorbed by skin. |
|
Fire Hazard |
Flash point data for 3-Nitroaniline are not available; however, 3-Nitroaniline is probably combustible. |
|
Synthesis |
1,3-Dinitrobenzene is added towarmwater containing magnesium sulfate. An aqueous solution of sodium hydrogen sulfide (6molar equivalents) is added gradually to the vigorously stirred emulsion, and reduction is completed by heating to 90℃. The 3-nitroaniline produced solidifies on cooling and is separated by filtration. |
|
Environmental fate |
Biological. A bacterial culture isolated from the Oconee River in North Georgia degraded 3- nitroaniline to the intermediate 4-nitrocatechol (Paris and Wolfe, 1987). A Pseudomonas sp. strain P6, isolated from a Matapeake silt loam, did not grow on 3-nitroaniline as the sole source of carbon. However, in the presence of 4-nitroaniline, all of the applied 3-nitroaniline metabolized completely to carbon dioxide (Zeyer and Kearney, 1983). In the presence of suspended natural populations from unpolluted aquatic systems, the second-order microbial transformation rate constant determined in the laboratory was reported to be 4.6 ± 0.1 x 10-13 L/organism?h (Steen, 1991). In activated sludge inoculum, following a 20-d adaptation period, no degradation was observed (Pitter, 1976). Chemical/Physical. Reacts with acids forming water soluble salts. |
|
Purification Methods |
Purify it as for o-nitroaniline. Warning: it is absorbed through the skin. [Beilstein 12 IV 1589.] |
|
Physical properties |
Yellow, rhombic crystals or powder. Finely dispersered particles form explosive mixtures. Combustible. |
|
General Description |
Yellow needles or yellow powder. |
InChI:InChI=1/C6H6N2O2/c9-8(10)7-6-4-2-1-3-5-6/h1-5,7H
99-09-2 Relevant articles
Support effect of Rh catalysts on the hydrogenation of m-dinitrobenzene
Martínez, José J.,Aguilera, Edna X.,Cubillos, Jairo,Rojas, Hugo,Gómez-Cortés, Antonio,Díaz, Gabriela
, p. 54 - 60 (2019)
The effect of the support (ZrO2, Al2O3, ...
Mesoporous nickel-aluminum mixed oxide: A promising catalyst in hydride-transfer reactions
Paul, Manidipa,Pal, Nabanita,Bhaumik, Asim
, p. 5129 - 5134 (2010)
The design and synthesis of a new nanost...
Carbon supported gold and silver: Application in the gas phase hydrogenation of m-dinitrobenzene
Cárdenas-Lizana, Fernando,De Pedro, Zahara M.,Gómez-Quero, Santiago,Kiwi-Minsker, Lioubov,Keane, Mark A.
, p. 138 - 146 (2015)
Abstract We have studied the gas phase c...
A PET-based fluorescent probe for monitoring labile Fe(ii) pools in macrophage activations and ferroptosis
Abedi, Syed Ali Abbas,Liu, Xiaogang,Lou, Kaiyan,Ma, Huijuan,Wang, Shanshan,Wang, Wei,Xing, Wanjin,Xu, Hang,Xu, Huan,Zhang, Xingchen
supporting information, p. 2979 - 2982 (2022/03/15)
A fluorescent probe (COU-LIP-1) for moni...
Au/Ni/Ni(OH)2/C Nanocatalyst with High Catalytic Activity and Selectivity for m-dinitrobenzene Hydrogenation
Ruan, Luna,Fu, Huan,Liao, Jianhua,Ding, Nengwen,Lan, Junjie,Yang, Kai,Rong, Mengke,Zhao, Ning,Zhu, Lihua,Chen, Bing Hui
, (2021/05/13)
The Au/Ni/Ni(OH)2/C bimetallic nanocatal...
Yeast supported gold nanoparticles: an efficient catalyst for the synthesis of commercially important aryl amines
Krishnan, Saravanan,Patel, Paresh N.,Balasubramanian, Kalpattu K.,Chadha, Anju
supporting information, p. 1915 - 1923 (2021/02/06)
Candida parapsilosisATCC 7330 supported ...
Aluminum Metal-Organic Framework-Ligated Single-Site Nickel(II)-Hydride for Heterogeneous Chemoselective Catalysis
Antil, Neha,Kumar, Ajay,Akhtar, Naved,Newar, Rajashree,Begum, Wahida,Dwivedi, Ashutosh,Manna, Kuntal
, p. 3943 - 3957 (2021/04/12)
The development of chemoselective and he...
99-09-2 Process route
-
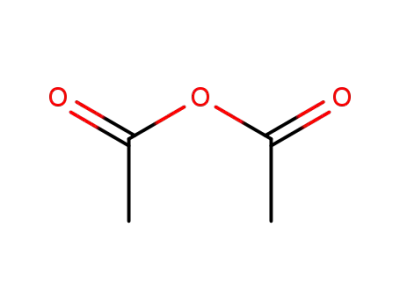
-
108-24-7
acetic anhydride

-
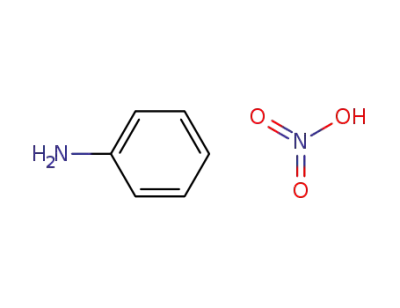
-
542-15-4
anilinium nitrate

-
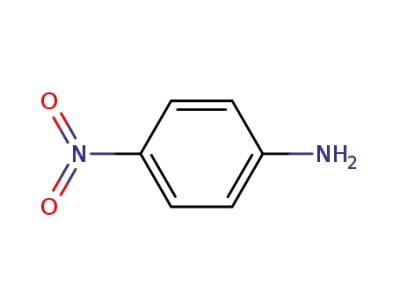
-
100-01-6,104810-17-5
4-nitro-aniline

-
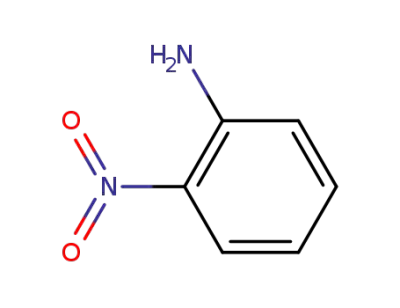
-
88-74-4
2-nitro-aniline

-
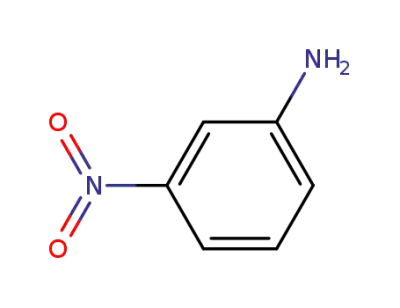
-
99-09-2
3-nitro-aniline
| Conditions | Yield |
|---|---|
|
at 0 ℃;
Product distribution;
|
-
-
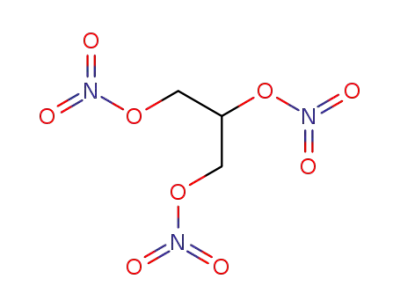
55-63-0,9010-02-0
glycerin trinitrate

-
-
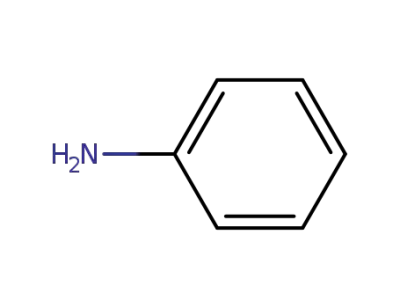
62-53-3
aniline

-

-
100-01-6,104810-17-5
4-nitro-aniline

-

-
99-09-2
3-nitro-aniline
| Conditions | Yield |
|---|---|
|
With
sulfuric acid;
|
99-09-2 Upstream products
-
99-65-0
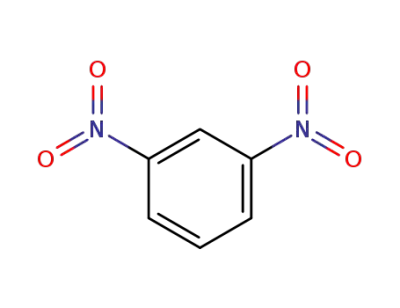
meta-dinitrobenzene
-
64-17-5
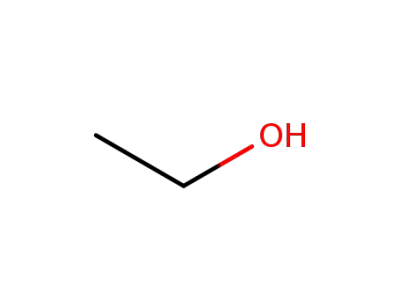
ethanol
-
13140-66-4
1-(3-nitrophenyl)-3-phenylthiourea
-
623-11-0
1-methyl-4-nitrosobenzene
99-09-2 Downstream products
-
24812-82-6
3-[N,N-Bis(2-hydroxyethyl)amino]nitrobenzene
-
102-38-5
N-(3-nitrophenyl)formamide
-
25233-49-2
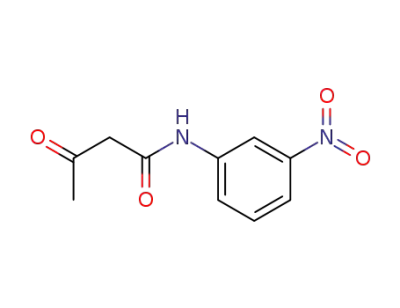
3'-nitroacetoacetanilide
-
32116-47-5
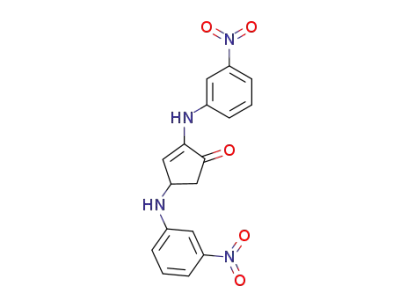
2,4-bis-(3-nitro-anilino)-cyclopent-2-enone
Relevant Products
-
2,5-Dimethoxy-Beta-Nitrostyrene
CAS:40276-11-7
-
2,4-Dinitrofluorobenzene
CAS:70-34-8
-
3,6-Dibromocarbazole
CAS:6825-20-3