668983-97-9
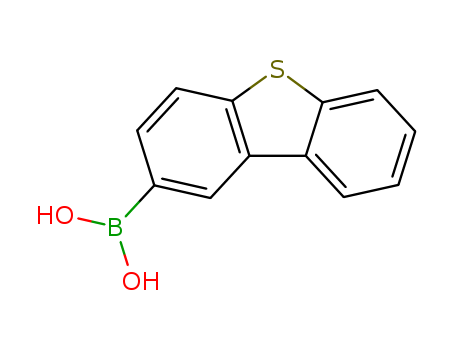
- Product Name:Dibenzothiophene-2-boronic Acid
- Molecular Formula:C12H9BO2S
- Purity:99%
- Molecular Weight:228.079
Product Details;
CasNo: 668983-97-9
Molecular Formula: C12H9BO2S
factory and supplier 668983-97-9 Dibenzothiophene-2-boronic Acid in stock
- Molecular Formula:C12H9BO2S
- Molecular Weight:228.079
- Vapor Pressure:4.97E-10mmHg at 25°C
- Refractive Index:1.738
- Boiling Point:480.2 °C at 760 mmHg
- PKA:8.39±0.30(Predicted)
- Flash Point:244.2 °C
- PSA:68.70000
- Density:1.38 g/cm3
- LogP:1.73430
DIBENZOTHIOPHENE-2-BORONIC ACID(Cas 668983-97-9) Usage
InChI:InChI=1/C12H9BO2S/c14-13(15)8-5-6-12-10(7-8)9-3-1-2-4-11(9)16-12/h1-7,14-15H
668983-97-9 Relevant articles
Electrochemical Synthesis of Biaryls via Oxidative Intramolecular Coupling of Tetra(hetero)arylborates
Music, Arif,Baumann, Andreas N.,Spie?, Philipp,Plantefol, Allan,Jagau, Thomas C.,Didier, Dorian
supporting information, p. 4341 - 4348 (2020/03/04)
We report herein versatile, transition m...
Bending-Type Electron Donor-Donor-Acceptor Triad: Dual Excited-State Charge-Transfer Coupled Structural Relaxation
Lin, Jia-An,Li, Shu-Wei,Liu, Zong-Ying,Chen, Deng-Gao,Huang, Chun-Ying,Wei, Yu-Chen,Chen, Yi-Yun,Tsai, Zheng-Hua,Lo, Chun-Yuan,Hung, Wen-Yi,Wong, Ken-Tsung,Chou, Pi-Tai
, p. 5981 - 5992 (2019/08/27)
The triad types of molecules with variou...
COMPOUND FOR ORGANIC ELECTRONIC ELEMENT, ORGANIC ELECTRONIC ELEMENT USING THE SAME, AND A ELECTRONIC DEVICE THEREOF
-
Paragraph 0142-0145, (2019/01/30)
The present invention provides a novel c...
NOVEL TRIAZINE COMPOUND, AND ORGANIC ELECTRONIC ELEMENT AND PLANT-GROWING LIGHTING THAT USE THE SAME
-
Paragraph 0076, (2018/07/28)
PROBLEM TO BE SOLVED: To provide a triaz...
668983-97-9 Process route
-
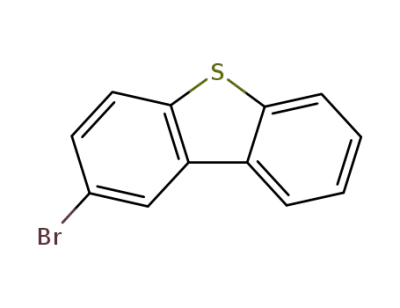
-
22439-61-8
2-bromodibenzothiophene

-
![dibenzo[b,d]thien-2-ylboronic acid](/upload/2026/1/b6be1590-903b-4c05-b985-4abbf648cb5a.png)
-
668983-97-9
dibenzo[b,d]thien-2-ylboronic acid
| Conditions | Yield |
|---|---|
|
2-bromodibenzothiophene;
With
n-butyllithium;
In
tetrahydrofuran; hexane; toluene;
at -70 ℃;
for 1h;
Inert atmosphere;
With
Triisopropyl borate;
In
tetrahydrofuran; hexane; toluene;
at 20 ℃;
for 6h;
With
hydrogenchloride;
In
tetrahydrofuran; hexane; water; toluene;
for 0.5h;
|
95% |
|
2-bromodibenzothiophene;
With
n-butyllithium;
In
tetrahydrofuran; hexane;
at -78 ℃;
for 1h;
Inert atmosphere;
With
Triisopropyl borate;
In
tetrahydrofuran; hexane;
at -78 - 20 ℃;
for 12h;
|
92% |
|
With
n-butyllithium; Trimethyl borate; sulfuric acid; water;
In
hexane;
at -78 - 20 ℃;
|
88% |
|
With
n-butyllithium; diethyl ether;
Behandeln des Reaktionsprodukts mit Triisopropylborat in Aether bei -60grad und Behandeln des Reaktionsgemisches mit Wasser;
|
-

-
22439-61-8
2-bromodibenzothiophene

-
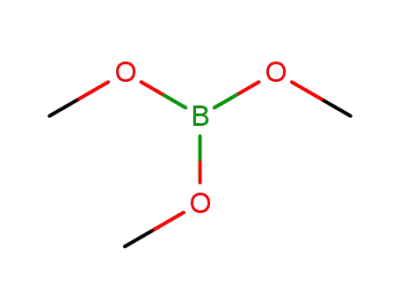
-
121-43-7,63156-11-6
Trimethyl borate

-
![dibenzo[b,d]thien-2-ylboronic acid](/upload/2026/1/b6be1590-903b-4c05-b985-4abbf648cb5a.png)
-
668983-97-9
dibenzo[b,d]thien-2-ylboronic acid
| Conditions | Yield |
|---|---|
|
2-bromodibenzothiophene;
With
n-butyllithium;
In
tetrahydrofuran; hexane;
at -78 - 0 ℃;
for 1h;
Trimethyl borate;
In
tetrahydrofuran; hexane;
at -78 - 20 ℃;
for 12h;
|
82% |
|
2-bromodibenzothiophene;
With
n-butyllithium;
In
tetrahydrofuran;
at -78 ℃;
for 1h;
Inert atmosphere;
Trimethyl borate;
In
tetrahydrofuran;
at 20 ℃;
for 3h;
Inert atmosphere;
With
hydrogenchloride;
In
tetrahydrofuran; water;
for 0.5h;
Inert atmosphere;
|
80% |
|
2-bromodibenzothiophene;
With
n-butyllithium;
In
tetrahydrofuran; hexane;
at -78 - 0 ℃;
for 1h;
Trimethyl borate;
In
tetrahydrofuran; hexane;
at -78 - 20 ℃;
for 12h;
|
|
|
2-bromodibenzothiophene;
With
n-butyllithium;
Trimethyl borate;
With
hydrogenchloride;
|
668983-97-9 Upstream products
-
22439-61-8

2-bromodibenzothiophene
-
132-65-0
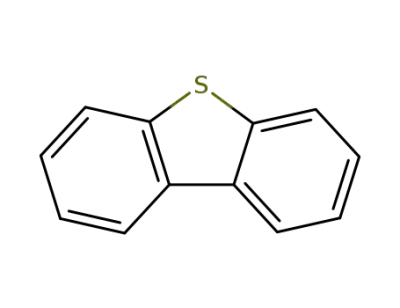
dibenzothiophene
-
5419-55-6
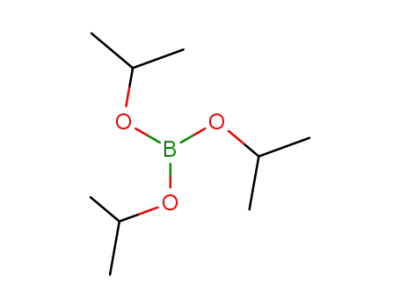
Triisopropyl borate
-
1776-66-5
triisopropylborane
668983-97-9 Downstream products
-
1398393-70-8
C44H27N3S
Relevant Products
-
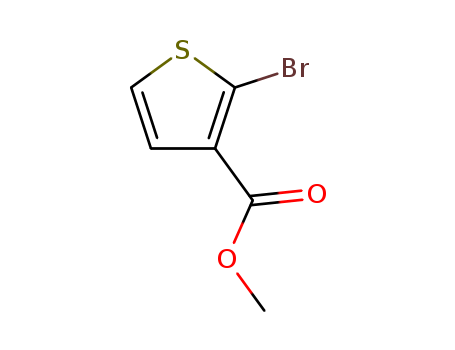
Methyl 2-bromothiophene-3-carboxylate
CAS:76360-43-5
-
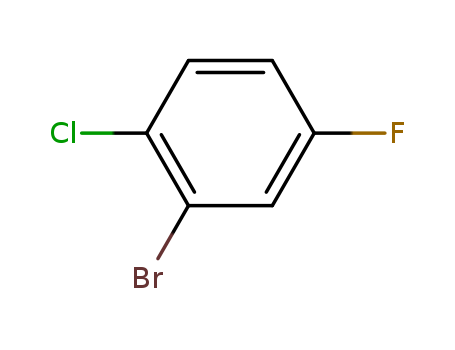
2-Bromo-1-chloro-4-fluorobenzene
CAS:201849-15-2