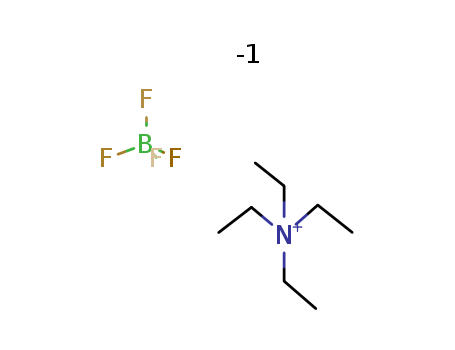
429-06-1
- Product Name:Tetraethylammonium tetrafluoroborate
- Molecular Formula:C8H20N.BF4
- Purity:99%
- Molecular Weight:217.058
Product Details;
CasNo: 429-06-1
Molecular Formula: C8H20N.BF4
Appearance: white crystals or crystalline powder
factory and manufacture 429-06-1 Tetraethylammonium tetrafluoroborate lonic liquid
- Molecular Formula:C8H20N.BF4
- Molecular Weight:217.058
- Appearance/Colour:white crystals or crystalline powder
- Vapor Pressure:0-0Pa at 20-25℃
- Melting Point:≥300 °C(lit.)
- PSA:0.00000
- Density:1.23 at 20℃
- LogP:3.18280
Tetraethylammonium tetrafluoroborate(Cas 429-06-1) Usage
|
General Description |
Visit our Sensor Applications portal to learn more. |
|
Purification Methods |
Dissolve the salt in hot MeOH, filter and add Et2O. It is soluble in ethylene chloride [Thompson & Kraus J Am Chem Soc 69 1016 1947, Wheeler & Sandstadt 77 2025 1955]. It has also been recrystallised three times from a mixture of ethyl acetate/hexane (5:1) or MeOH/pet ether, then stored at 95o for 48hours under vacuum [Henry & Faulkner J Am Chem Soc 107 3436 1985, Huang et al. Anal Chem 58 2889 1986]. It is used as a supporting electrolyte. [Beilstein 4 IV 333.] |
InChI:InChI=1/C8H20N.BF4/c1-5-9(6-2,7-3)8-4;2-1(3,4)5/h5-8H2,1-4H3;/q+1;-1
429-06-1 Relevant articles
Electrochemistry as an attractive and effective tool for the synthesis and immobilization of porphyrins on an electrode surface
Hebié, Seydou,Dimé, Abdou K. D.,Devillers, Charles H.,Lucas, Dominique
, p. 8281 - 8289 (2015)
Magnesium(II) 10-phenyl-5,15-p-ditolylpo...
Synthesis and characterization of {Ni(NO)}10 and {Co(NO) 2}10 complexes supported by thiolate ligands
Tennyson, Andrew G.,Dhar, Shanta,Lippard, Stephen J.
, p. 15087 - 15098 (2008)
Nitric oxide is an important molecule in...
Towards sustainable synthesis of pyren-1-yl azoliums via electrochemical oxidative C-N coupling
De Robillard, Guillaume,Makni, Oumayma,Cattey, Hélène,Andrieu, Jacques,Devillers, Charles H.
, p. 4669 - 4679 (2015)
Electrosynthesis of 1-methyl-3-(pyren-1-...
Electrochemical phosphorylation of coumarins catalyzed by transition metal complexes (Ni—Mn, Co—Mn)
Strekalova,Khrizanforov,Gryaznova,Khrizanforova,Budnikova, Yu. H.
, p. 1295 - 1298 (2016)
A possibility of electrochemical phospho...
The influence of temperature and concentration on viscous flow of solutions of Et4NBF4 in propylene carbonate
Afanas'ev,Tyunina, E. Yu.,Chekunova
, p. 2069 - 2073 (2009)
Solutions of tetraethylammonium tetraflu...
Mechanism of the Platinum(II)-Catalyzed Hydroamination of 4-Pentenylamines
Bender, Christopher F.,Brown, Timothy J.,Widenhoefer, Ross A.
, p. 113 - 125 (2016)
The mechanism of the platinum(II)-cataly...
Electrophilic additions of metal fragments containing 11- and 12-group elements to the anion carbide cluster [Fe5MoC(CO)17]2-. X-ray crystal structures of (NEt4)[Fe5MoAuC(CO)17(PMe3)] and [Fe5MoAu2C(CO)17(dppm)]
Reina, Roser,Rodríguez, Laura,Rossell, Oriol,Seco, Miquel,Font-Bardia, Mercè,Solans, Xavier
, p. 1575 - 1579 (2001)
The reaction of (NEt4)2[Fe5MoC(CO)17] wi...
Fe and Ni-catalyzed electrochemical perfluoroalkylation of C—H bonds of coumarins
Khrizanforov,Strekalova,Grinenko,Khrizanforova,Gryaznova,Budnikova, Yu. H.
, p. 1446 - 1449 (2017)
A new method for the preparation of perf...
A four-ethyl four simple method for preparing ammonium borofluoride (by machine translation)
-
Paragraph 0021-0042; 0045-0052, (2018/10/11)
The invention discloses an electronic po...
A general diastereoselective synthesis of highly functionalized ferrocenyl ambiphiles enabled on a large scale by electrochemical purification
Lerayer, Emmanuel,Renaut, Patrice,Roger, Julien,Pirio, Nadine,Cattey, Hélène,Devillers, Charles H.,Lucas, Dominique,Hierso, Jean-Cyrille
supporting information, p. 6017 - 6020 (2017/07/11)
A general synthesis of highly functional...
429-06-1 Process route
-
-
56-34-8
tetraethylammonium chloride

-
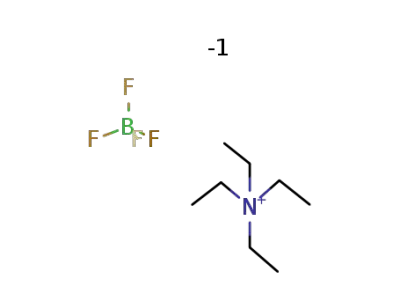
-
429-06-1
N,N,N,N-tetraethylammonium tetrafluoroborate
| Conditions | Yield |
|---|---|
|
With
potassium tetrafluoroborate;
In
acetonitrile;
at 20 ℃;
for 0.25h;
|
-
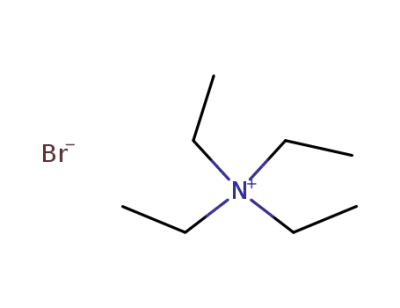
-
71-91-0
tetraethylammonium bromide

-
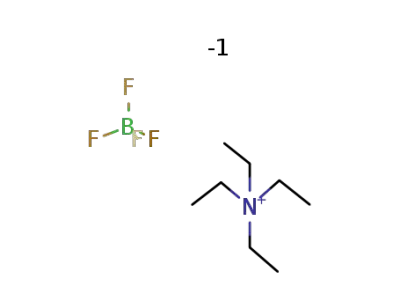
-
429-06-1
N,N,N,N-tetraethylammonium tetrafluoroborate
| Conditions | Yield |
|---|---|
|
With
tetrafluoroboric acid;
In
methanol;
for 0.5h;
|
|
|
With
tetrafluoroboric acid;
|
429-06-1 Upstream products
-
64-67-5
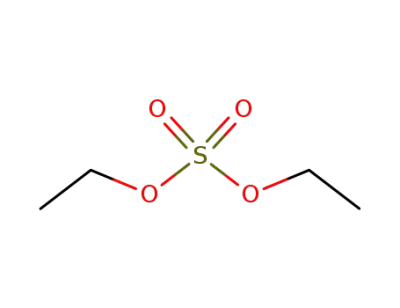
diethyl sulfate
-
121-44-8

triethylamine
-
74-96-4
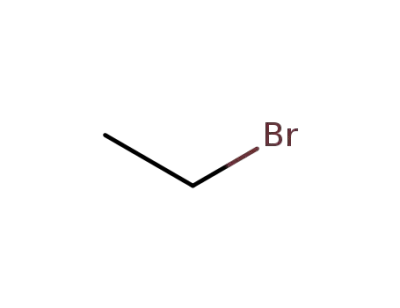
ethyl bromide
-
68-05-3
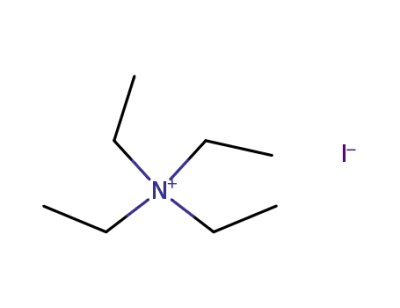
tetraethylammonium iodide
429-06-1 Downstream products
-
7242-58-2
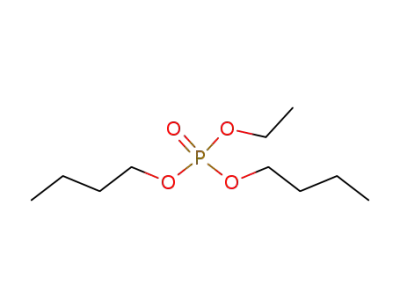
phosphoric acid dibutyl ester ethyl ester
-
121-44-8

triethylamine
-
114526-94-2
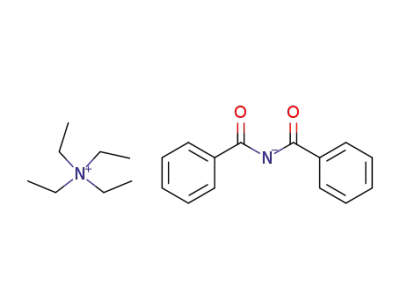
tetraethylammonium salt of dibenzoylimide
-
114526-96-4
tetraethylammonium salt of p-cyanoformanilide
Relevant Products
-
1-Butyl-3-methylimidazolium tetrafluoroborate
CAS:174501-65-6
-
1-Hexyl-3-methylimidazolium bis((trifluoromethyl)sulfonyl)imide
CAS:916729-96-9
-
1,1'-Bis(di-tert-butylphosphino)ferrocene
CAS:84680-95-5