461-82-5
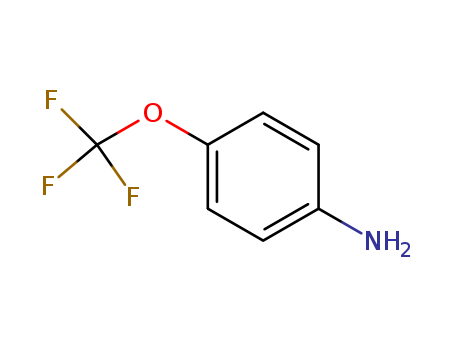
- Product Name:4-(Trifluoromethoxy)aniline
- Molecular Formula:C7H6F3NO
- Purity:99%
- Molecular Weight:177.126
Product Details;
CasNo: 461-82-5
Molecular Formula: C7H6F3NO
Appearance: Clear yellow liquid
factory and supplier 461-82-5 4-(Trifluoromethoxy)aniline in stock
- Molecular Formula:C7H6F3NO
- Molecular Weight:177.126
- Appearance/Colour:Clear yellow liquid
- Vapor Pressure:2.97mmHg at 25°C
- Refractive Index:1.4630
- Boiling Point:192.4 °C at 760 mmHg
- PKA:3.75±0.10(Predicted)
- Flash Point:80.6 °C
- PSA:35.25000
- Density:1.34 g/cm3
- LogP:2.74860
4-(Trifluoromethoxy)aniline(Cas 461-82-5) Usage
InChI:InChI:1S/C7H6F3NO/c8-7(9,10)12-6-3-1-5(11)2-4-6/h1-4H,11H2
461-82-5 Relevant articles
Cyclic (Alkyl)(amino)carbene Ligand-Promoted Nitro Deoxygenative Hydroboration with Chromium Catalysis: Scope, Mechanism, and Applications
Zhao, Lixing,Hu, Chenyang,Cong, Xuefeng,Deng, Gongda,Liu, Liu Leo,Luo, Meiming,Zeng, Xiaoming
supporting information, p. 1618 - 1629 (2021/01/25)
Transition metal catalysis that utilizes...
Minimization of Back-Electron Transfer Enables the Elusive sp3 C?H Functionalization of Secondary Anilines
Zhao, Huaibo,Leonori, Daniele
supporting information, p. 7669 - 7674 (2021/03/08)
Anilines are some of the most used class...
Method for continuous hydrogenation preparation of aromatic amine through nitro-compound
-
Paragraph 0046-0047, (2019/06/27)
The invention provides a method for cont...
Method for synthesizing insecticide metaflumizone intermediate p-trifluoromethoxy aniline
-
Paragraph 0025-0049, (2019/01/23)
The invention discloses a method for syn...
461-82-5 Process route
-
-
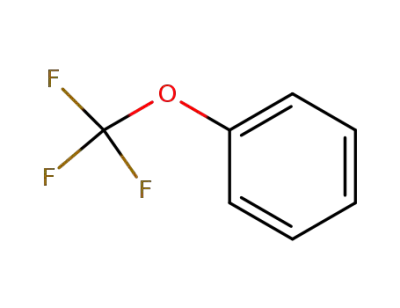
456-55-3
1-trifluoromethoxybenzene

-
-
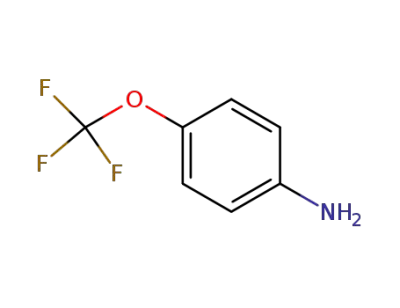
461-82-5
4-(trifluoromethoxy)aniline
| Conditions | Yield |
|---|---|
|
1-trifluoromethoxybenzene;
With
sodium ferrate(VI); sodium bromide;
In
dimethyl sulfoxide;
at 95 ℃;
for 4h;
Inert atmosphere;
With
sodium amide;
In
dimethyl sulfoxide;
at 155 ℃;
for 10h;
under 3040.2 Torr;
Solvent;
Temperature;
Reagent/catalyst;
Pressure;
Inert atmosphere;
|
98.2% |
|
Multi-step reaction with 2 steps
1: sulfuric acid; nitric acid / dichloromethane / 2 h / 0 - 30 °C
2: iron; hydrogenchloride / methanol / 2 h / 60 - 65 °C
With
hydrogenchloride; sulfuric acid; nitric acid; iron;
In
methanol; dichloromethane;
|
-
-
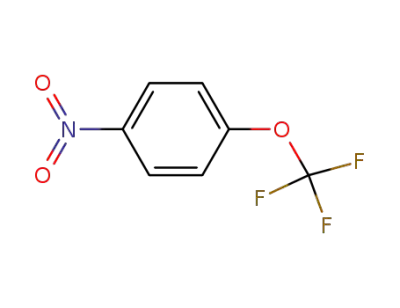
713-65-5
4-nitrophenyl trifluoromethyl ether

-

-
461-82-5
4-(trifluoromethoxy)aniline
| Conditions | Yield |
|---|---|
|
With
C36H56Cl3CrN2O; magnesium; 4,4,5,5-tetramethyl-[1,3,2]-dioxaboralane;
In
tetrahydrofuran;
at 60 ℃;
for 24h;
Inert atmosphere;
|
92% |
|
With
hydrogen;
ShPAK-0.5;
In
methanol;
at 44 - 46 ℃;
under 29420.3 - 30891.3 Torr;
|
89% |
|
With
hydrogenchloride; iron;
In
methanol;
at 60 - 65 ℃;
for 2h;
|
|
|
With
hydrogen;
In
ethanol;
at 40 ℃;
for 0.833333h;
Autoclave;
|
|
|
With
hydrogen;
at 85 - 95 ℃;
for 6h;
under 18751.9 - 30003 Torr;
|
|
|
With
hydrogen;
In
methanol;
under 750.075 Torr;
|
|
|
With
hydrogen;
In
methanol;
under 750.075 Torr;
|
461-82-5 Upstream products
-
456-71-3
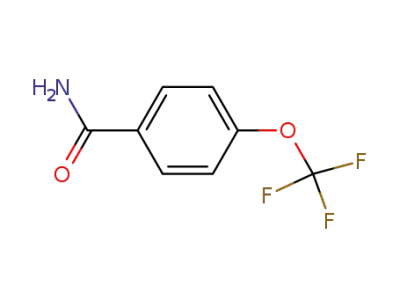
4-trifluoromethoxybenzamide
-
713-65-5

4-nitrophenyl trifluoromethyl ether
-
36823-89-9
4-(Trichloromethoxy)benzoyl chloride
-
332-25-2
4-(trifluoromethoxy)benzonitrile
461-82-5 Downstream products
-
64628-43-9
1-(2-Methyl-benzoyl)-3-(4-trifluoromethoxy-phenyl)-urea
-
68105-65-7
(2,4-Dinitro-naphthalen-1-yl)-(4-trifluoromethoxy-phenyl)-amine
-
72773-48-9
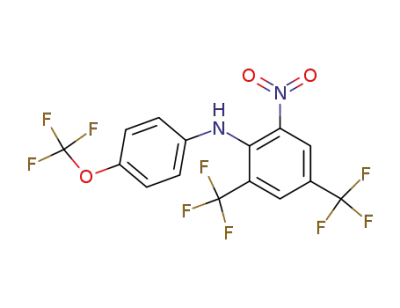
(2-Nitro-4,6-bis-trifluoromethyl-phenyl)-(4-trifluoromethoxy-phenyl)-amine
-
64628-45-1
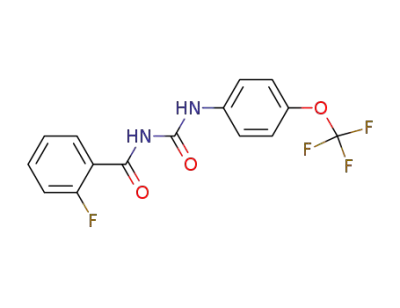
1-(2-Fluoro-benzoyl)-3-(4-trifluoromethoxy-phenyl)-urea
Relevant Products
-
1-Butyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide
CAS:174899-83-3
-
Decanoic Anhydride
CAS:2082-76-0
-
2,2,2-trifluoroethyl acrylate
CAS:407-47-6