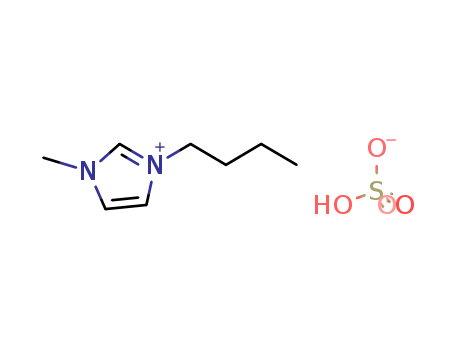
262297-13-2
- Product Name:3-Butyl-1-methyl-1H-imidazol-3-ium hydrogen sulfate
- Molecular Formula:C8H16N2O4S
- Purity:99%
- Molecular Weight:236.292
Product Details;
CasNo: 262297-13-2
Molecular Formula: C8H16N2O4S
factory and manufacture 262297-13-2 3-Butyl-1-methyl-1H-imidazol-3-ium hydrogen sulfate lonic liquid
- Molecular Formula:C8H16N2O4S
- Molecular Weight:236.292
- Melting Point:29-32 °C
- Flash Point:284 °C
- PSA:94.62000
- Density:1.27g/ml
- LogP:1.19810
1-Butyl-3-methylimidazolium hydrogensulfate(Cas 262297-13-2) Usage
|
General Description |
1-Butyl-3-methylimidazolium hydrogensulfate ([Bmim]HSO4) is an ionic liquid that serves as an efficient, environmentally friendly catalyst and solvent in the synthesis of biologically active thiochromeno[3,4-d]pyrimidine derivatives. It enables high-yielding reactions with short reaction times, contributing to the development of compounds with notable antibacterial, antibiofilm, and ROS-inducing properties, particularly against pathogens like *Staphylococcus aureus* and *Bacillus subtilis*. |
InChI:InChI=1/C8H15N2.H2O4S/c1-3-4-5-10-7-6-9(2)8-10;1-5(2,3)4/h6-8H,3-5H2,1-2H3;(H2,1,2,3,4)/q+1;/p-1
262297-13-2 Relevant articles
Concentration-dependent apparent partition coefficients of ionic liquids possessing ethyl- and bi-sulphate anions
Jain, Preeti,Kumar, Anil
, p. 1105 - 1113 (2016)
This study deals with the concentration ...
Fine tuning the ionic liquid-vacuum outer atomic surface using ion mixtures
Villar-Garcia, Ignacio J.,Fearn, Sarah,Ismail, Nur L.,McIntosh, Alastair J. S.,Lovelock, Kevin R. J.
, p. 5367 - 5370 (2015)
Ionic liquid-vacuum outer atomic surface...
Choline chloride-thiourea, a deep eutectic solvent for the production of chitin nanofibers
Mukesh, Chandrakant,Mondal, Dibyendu,Sharma, Mukesh,Prasad, Kamalesh
, p. 466 - 471 (2014)
Deep eutectic solvents (DESs) consisting...
Are alkyl sulfate-based protic and aprotic ionic liquids stable with water and alcohols? A thermodynamic approach
Jacquemin, Johan,Goodrich, Peter,Jiang, Wei,Rooney, David W.,Hardacre, Christopher
, p. 1938 - 1949 (2013)
The knowledge of the chemical stability ...
A quick, simple, robust method to measure the acidity of ionic liquids
Gr?svik, John,Hallett, Jason P.,To, Trang Quynh,Welton, Tom
, p. 7258 - 7261 (2014)
Introduced here is a quick, simple, robu...
Oxidative desulfurization of gasoline by ionic liquids coupled with extraction by organic solvents
Abro, Rashid,Gao, Shurong,Chen, Xiaochun,Yu, Guangren,Abdeltawab Salem, Ahmed A.,Al-Deyabb
, p. 998 - 1006 (2016)
In this work, desulfurization of real fl...
Efficient combination of recyclable task specific ionic liquid and microwave dielectric heating for the synthesis of lipophilic esters
Arfan, Atef,Bazureau, Jean Pierre
, p. 743 - 748 (2005)
Mild and efficient esterification reacti...
Influence of anions of imidazole ionic liquids on dissolution of cellulose
Liu,Yu,Zhou,Zhang,Zhang
, p. 8266 - 8270 (2013)
[Bmim]Cl, [Bmim]Br, [Bmim]HSO4, [Bmim]BF...
Development of Acidic Imidazolium Ionic Liquids for Activation of Kraft Lignin by Controlled Oxidation: Comprehensive Evaluation and Practical Utility
Klapiszewski, ?ukasz,Szalaty, Tadeusz J.,Kurc, Beata,Stanisz, Ma?gorzata,Zawadzki, Bartosz,Skrzypczak, Andrzej,Jesionowski, Teofil
, p. 361 - 374 (2018)
A novel, eco-friendly method for the act...
The efficient hydroxyalkylation of phenol with formaldehyde to bisphenol F over a thermoregulated phase-separable reaction system containing a water-soluble Bronsted acidic ionic liquid
Wang, Qing,Wu, Zhi Min,Li, Yongfei,Tan, Ying,Liu, Ning,Liu, Yuejin
, p. 33466 - 33473 (2014)
The efficient hydroxyalkylation of pheno...
Kinetics and quantum chemical study for cyclotrimerization of propanal catalyzed by Br?nsted acidic ionic liquids
Wu, Li,Li, Zhen,Wang, Fang,Lei, Min,Chen, Jing
, p. 86 - 93 (2013)
Several Br?nsted acidic ionic liquids (B...
Theoretical and experimental comparative study of nonlinear properties of imidazolium cation based ionic liquids
Ferreira, Vinícius Castro,Zanchet, Letícia,Monteiro, Wesley Formentin,da Trindade, Letícia Guerreiro,de Souza, Michèle Oberson,Correia, Ricardo Rego Bordalo
, (2021/02/09)
This work describes the experimental and...
Bismuth mediated barbier synthesis of α-homoallylic alcohols via a sigmatropic rearrangement in [bmim][HSO4]
Chatterjee, Sucheta,Dey, Papiya,Kanojia, Seema V.,Chattopadhyay, Subrata,Goswami, Dibakar
supporting information, p. 765 - 775 (2020/12/13)
A novel protocol for the Bismuth metal m...
Insights on the catalytic behaviour of sulfonic acid-functionalized ionic liquids (ILs) in transesterification reactions - voltammetric characterization of sulfonic task-specific ILs with bisulfate anions
Martini, María B.,Fernández, José L.,Adam, Claudia G.
, p. 2731 - 2741 (2021/02/12)
This work shows for the first time the l...
262297-13-2 Process route
-

-
85100-77-2
1-n-butyl-3-methylimidazolim bromide

-
-
262297-13-2
1-butyl-3-methylimidazolium hydrogen sulfate
| Conditions | Yield |
|---|---|
|
With
1-hexene; sulfuric acid; dihydrogen peroxide;
at 25 ℃;
for 3h;
|
97% |
|
With
sulfuric acid; silver sulfate;
In
ethanol;
at 50 ℃;
for 2h;
|
75% |
|
With
sulfuric acid; silver sulfate;
In
ethanol; water;
at 50 ℃;
for 2h;
|
75% |
|
With
sodium hydrogen sulfate;
at 20 ℃;
for 72h;
|
|
|
With
sulfuric acid;
at 20 ℃;
for 24h;
|
|
|
With
sodium hydrogen sulfate;
In
acetone;
at 20 ℃;
for 24h;
|
|
|
With
sulfuric acid;
In
dichloromethane;
at 40 ℃;
|
|
|
With
sulfuric acid;
In
dichloromethane;
at 20 ℃;
for 24h;
|
|
|
With
sulfuric acid;
In
dichloromethane;
at 0 ℃;
for 26h;
Reflux;
|
|
|
With
sodium hydrogen sulfate;
In
methanol;
at 20 ℃;
for 24h;
|
|
|
With
sodium hydrogen sulfate;
In
methanol; water;
at 20 ℃;
for 24h;
|
|
|
With
sulfuric acid;
In
dichloromethane;
for 48h;
Reflux;
|
4.58 g |
|
With
sodium hydrogen sulfate;
In
methanol;
at 68 ℃;
for 72h;
|
-
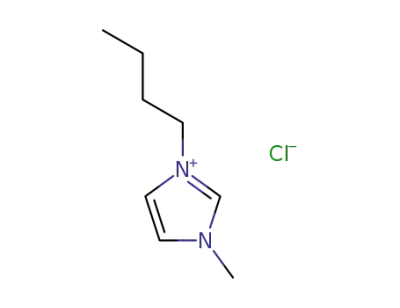
-
79917-90-1
1-butyl-3-methylimidazolium chloride

-

-
262297-13-2
1-butyl-3-methylimidazolium hydrogen sulfate
| Conditions | Yield |
|---|---|
|
With
sulfuric acid;
In
dichloromethane;
for 48h;
Reflux;
Cooling with ice;
|
99.4% |
|
With
sodium hydrogen sulfate;
In
acetonitrile;
at 25 ℃;
for 96h;
Inert atmosphere;
|
97% |
|
With
sulfuric acid;
In
dichloromethane;
for 24h;
Reflux;
|
95% |
|
With
sodium hydrogen sulfate;
In
acetone;
at 20 ℃;
for 24h;
|
89% |
|
With
sulfuric acid;
In
dichloromethane;
|
|
|
With
sulfuric acid;
In
dichloromethane;
Cooling;
Reflux;
|
|
|
With
sulfuric acid;
|
|
|
With
sulfuric acid;
In
dichloromethane;
at 70 ℃;
for 24h;
|
|
|
With
sulfuric acid;
In
dichloromethane;
|
|
|
With
sulfuric acid;
In
dichloromethane;
at 20 ℃;
for 24h;
Inert atmosphere;
|
|
|
Multi-step reaction with 2 steps
1: Dowex Monosphere 550 A UPW OH form resin / water
2: sulfuric acid / water
With
sulfuric acid;
In
water;
|
|
|
With
sulfuric acid;
In
water;
at 100 ℃;
for 4h;
|
262297-13-2 Upstream products
-
65039-05-6
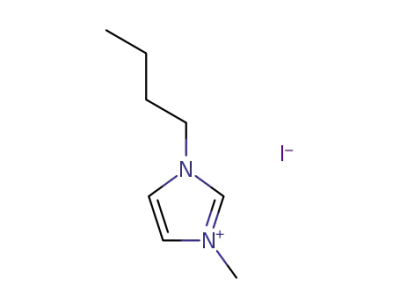
1-methyl-3-(n-butyl)imidazolium iodide
-
79917-90-1

1-butyl-3-methylimidazolium chloride
-
85100-77-2

1-n-butyl-3-methylimidazolim bromide
-
4316-42-1
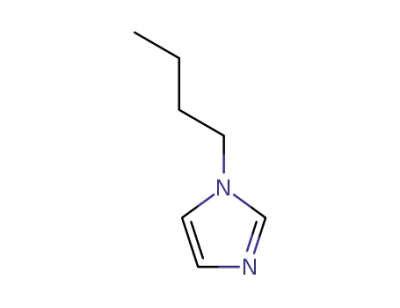
1-Butylimidazole
Relevant Products
-
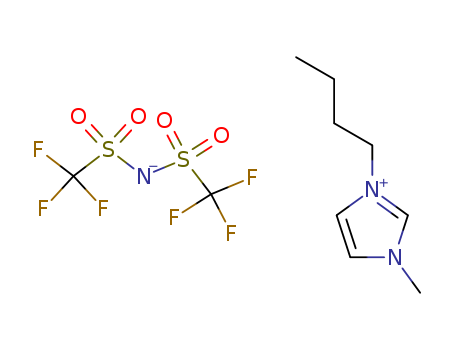
1-Butyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide
CAS:174899-83-3
-
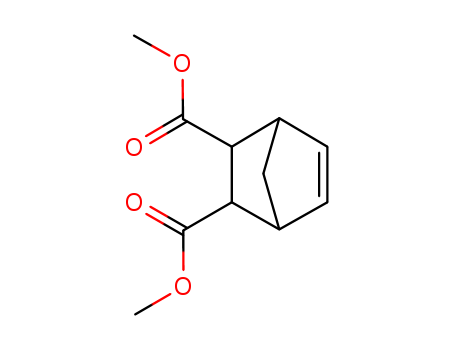
Dimethyl 5-norbornene-2,3-dicarboxylate
CAS:5826-73-3
-
1-Ethyl-1-methylpyrrolidinium bis(trifluoromethylsulfonyl)imide
CAS:223436-99-5