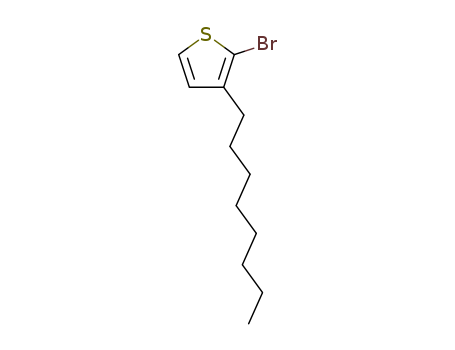
145543-83-5
- Product Name:2-bromo-3-octylthiophene
- Molecular Formula:C12H19BrS
- Purity:99%
- Molecular Weight:275.253
Product Details;
CasNo: 145543-83-5
Molecular Formula: C12H19BrS
factory and supplier 145543-83-5 2-bromo-3-octylthiophene in stock
- Molecular Formula:C12H19BrS
- Molecular Weight:275.253
- Vapor Pressure:0.001mmHg at 25°C
- Refractive Index:1.521
- Boiling Point:307.088 °C at 760 mmHg
- Flash Point:139.522 °C
- PSA:28.24000
- Density:1.213 g/cm3
- LogP:5.41360
2-bromo-3-octylthiophene(Cas 145543-83-5) Usage
InChI:InChI=1/C12H19BrS/c1-2-3-4-5-6-7-8-11-9-10-14-12(11)13/h9-10H,2-8H2,1H3
145543-83-5 Relevant articles
Effect of molecular weight on electronic, electrochemical and spectroelectrochemical properties of poly(3,3″-dioctyl-2,2′5′, 2″-terthiophene)
Pokrop, Rafal,Verilhac, Jean-Marie,Gasior, Anna,Wielgus, Ireneusz,Zagorska, Malgorzata,Travers, Jean-Pierre,Pron, Adam
, p. 3099 - 3106 (2006)
Poly(3,3″-dioctyl-2,2′5′,2″-terthiophene...
Tuning the Solid State Emission of Thin Films/Microspheres Obtained from Alternating Oligo(3-octylthiophenes) and 2,6-Bis(pyrazole)pyridine Copolymers by Varying Conjugation Length and Eu3+/Tb3+ Metal Coordination
Narayana, Yemineni S. L. V.,Baumgarten, Martin,Müllen, Klaus,Chandrasekar, Rajadurai
, p. 4801 - 4812 (2015)
A series of dialkynyl-functionalized oli...
Medium band gap conjugated polymers from thienoacene derivatives and pentacyclic aromatic lactam as promising alternatives of poly(3-hexylthiophene) in photovoltaic application
Gao, Peili,Tong, Junfeng,Guo, Pengzhi,Li, Jianfeng,Wang, Ningning,Li, Cheng,Ma, Xuying,Zhang, Peng,Wang, Chenglong,Xia, Yangjun
, p. 85 - 95 (2018)
Two alternating medium band gap conjugat...
1H and13C NMR study of regioregular head-to-tail oligo(octylthiophene)s and poly(octylthiophene)
Bras, Jerome,Pepin-Donat, Brigitte
, p. 57 - 67 (2001)
The 1H and 13C signals of the aromatic r...
Organic Thin-film Solar Cells Using Benzotrithiophene Derivatives Bearing Acceptor Units as Non-Fullerene Acceptors
Matsumoto, Kouichi,Yamashita, Kazuhiro,Sakoda, Yuuki,Ezoe, Hinata,Tanaka, Yuki,Okazaki, Tatsuya,Ohkita, Misaki,Tanaka, Senku,Aoki, Yuki,Kiriya, Daisuke,Kashimura, Shigenori,Maekawa, Masahiko,Kuroda-Sowa, Takayoshi,Okubo, Takashi
, p. 4620 - 4629 (2021/09/10)
New star-shaped non-fullerene acceptors ...
Thiophene-benzothiadiazole based donor–acceptor–donor (D-A-D) bolaamphiphiles, self-assembly and photophysical properties
Chang, Qing,Cheng, Xiaohong,Ding, Wei,Ma, Tao,Zhang, Lin
, (2021/11/03)
Bolaamphiphilies with D-A-D type π-conju...
Synthesis and characterization of new D-π-A and A-π-D-π-A type oligothiophene derivatives
Pandolfi, Fabiana,Rocco, Daniele,Mattiello, Leonardo
supporting information, p. 3018 - 3025 (2019/03/21)
In this work, we present a series of new...
TRIPHENYLAMINE COMPOUNDS, POLYMERS MADE THEREFROM AND ELECTROCHROMIC DEVICE COMPRISING THE SAME
-
Paragraph 0196; 0199; 0204-0206, (2018/05/03)
The present invention relates to a triph...
145543-83-5 Process route
-

-
65016-62-8,104934-51-2
3-octylthiophene

-
-
145543-83-5
2-bromo-3-octylthiophene
| Conditions | Yield |
|---|---|
|
With
N-Bromosuccinimide;
In
N,N-dimethyl-formamide;
at 0 - 20 ℃;
Darkness;
|
99% |
|
With
N-Bromosuccinimide; acetic acid;
at 15 ℃;
for 2h;
|
98% |
|
With
N-Bromosuccinimide;
In
tetrahydrofuran;
Inert atmosphere;
|
98% |
|
With
N-Bromosuccinimide; acetic acid;
In
chloroform;
at 0 ℃;
for 0.5h;
Inert atmosphere;
|
97.9% |
|
With
N-Bromosuccinimide;
In
acetic acid;
for 0.166667h;
|
94.2% |
|
With
N-Bromosuccinimide;
In
chloroform; acetic acid;
at 0 ℃;
|
94.6% |
|
With
N-Bromosuccinimide;
In
N,N-dimethyl-formamide;
at 5 - 25 ℃;
|
93% |
|
With
N-Bromosuccinimide;
In
chloroform; acetic acid;
Ambient temperature;
|
91% |
|
3-octylthiophene;
With
N-Bromosuccinimide;
In
chloroform;
at 20 ℃;
for 4h;
With
N-Bromosuccinimide;
In
chloroform;
for 4h;
Heating;
|
90% |
|
With
N-Bromosuccinimide;
In
N,N-dimethyl-formamide;
at 20 ℃;
for 12h;
|
90% |
|
With
N-Bromosuccinimide;
In
N,N-dimethyl-formamide;
at 20 ℃;
Cooling with ice;
|
89% |
|
With
N-Bromosuccinimide;
In
N,N-dimethyl-formamide;
at 20 ℃;
Cooling with ice;
|
89% |
|
With
N-Bromosuccinimide;
In
chloroform; acetic acid;
at 0 ℃;
|
85% |
|
With
N-Bromosuccinimide;
In
chloroform; acetic acid;
|
82% |
|
With
N-Bromosuccinimide; acetic acid;
In
chloroform;
at 20 ℃;
for 3h;
|
81% |
|
With
N-Bromosuccinimide;
In
N,N-dimethyl-formamide;
for 2h;
Ambient temperature;
|
79% |
|
With
bromine;
In
tetrachloromethane;
at -20 ℃;
for 2h;
|
75% |
|
With
N-Bromosuccinimide;
In
N,N-dimethyl-formamide;
for 1.25h;
|
65.8% |
|
With
N-Bromosuccinimide;
In
chloroform; acetic acid;
|
60% |
|
With
bromine;
In
acetic acid;
at 0 ℃;
for 0.5h;
|
40% |
|
With
N-Bromosuccinimide;
In
chloroform; acetic acid;
|
|
|
With
N-Bromosuccinimide;
In
chloroform;
|
|
|
With
N-Bromosuccinimide;
In
chloroform; acetic acid;
|
|
|
With
N-Bromosuccinimide;
In
tetrahydrofuran;
at 20 ℃;
|
|
|
With
N-Bromosuccinimide;
In
tetrahydrofuran;
at 25 ℃;
for 15h;
|
|
|
With
N-Bromosuccinimide; acetic acid;
In
chloroform;
|
|
|
With
N-Bromosuccinimide;
In
N,N-dimethyl-formamide;
|
|
|
With
N-Bromosuccinimide;
In
N,N-dimethyl-formamide;
at 20 ℃;
for 3h;
Inert atmosphere;
|
14.4 g |
|
With
N-Bromosuccinimide;
In
tetrahydrofuran;
at 0 - 5 ℃;
for 15h;
|
|
|
With
N-Bromosuccinimide;
In
tetrahydrofuran;
at 20 ℃;
|
-
-
17049-49-9
octylmagnesium bromide

-

-
145543-83-5
2-bromo-3-octylthiophene
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 2 steps
1: 61 percent / Ni(dppp)Cl2 / diethyl ether / 35 °C
2: 40 percent / Br2 / acetic acid / 0.5 h / 0 °C
With
1,3-bis[(diphenylphosphino)propane]dichloronickel(II); bromine;
In
diethyl ether; acetic acid;
|
|
|
Multi-step reaction with 2 steps
1: 1,3-bis[(diphenylphosphino)propane]dichloronickel(II) / tetrahydrofuran / 15 h / Reflux
2: N-Bromosuccinimide / tetrahydrofuran / 15 h / 25 °C
With
N-Bromosuccinimide; 1,3-bis[(diphenylphosphino)propane]dichloronickel(II);
In
tetrahydrofuran;
1: |Kumada Cross-Coupling;
|
|
|
Multi-step reaction with 2 steps
1: 1,3-bis[(diphenylphosphino)propane]dichloronickel(II) / diethyl ether / 0.25 h / 0 - 5 °C / Inert atmosphere
2: N-Bromosuccinimide; acetic acid / chloroform / 0.5 h / 0 °C / Inert atmosphere
With
N-Bromosuccinimide; 1,3-bis[(diphenylphosphino)propane]dichloronickel(II); acetic acid;
In
diethyl ether; chloroform;
1: |Kumada Cross-Coupling;
|
|
|
Multi-step reaction with 2 steps
1: 1,3-bis[(diphenylphosphino)propane]dichloronickel(II) / tetrahydrofuran / 15 h / Reflux
2: N-Bromosuccinimide / tetrahydrofuran / 15 h / 0 - 5 °C
With
N-Bromosuccinimide; 1,3-bis[(diphenylphosphino)propane]dichloronickel(II);
In
tetrahydrofuran;
1: |Kumada Cross-Coupling;
|
|
|
Multi-step reaction with 2 steps
1: 1,3-bis[(diphenylphosphino)propane]dichloronickel(II) / 0 - 20 °C / Inert atmosphere
2: N-Bromosuccinimide / tetrahydrofuran / Inert atmosphere
With
N-Bromosuccinimide; 1,3-bis[(diphenylphosphino)propane]dichloronickel(II);
In
tetrahydrofuran;
1: |Kumada Cross-Coupling;
|
|
|
Multi-step reaction with 2 steps
1: 1,3-bis[(diphenylphosphino)propane]dichloronickel(II) / tetrahydrofuran / Reflux
2: N-Bromosuccinimide / tetrahydrofuran / 20 °C
With
N-Bromosuccinimide; 1,3-bis[(diphenylphosphino)propane]dichloronickel(II);
In
tetrahydrofuran;
|
145543-83-5 Upstream products
-
65016-62-8

3-octylthiophene
-
872-31-1
3-Bromothiophene
-
17049-49-9
octylmagnesium bromide
-
111-83-1
1-bromo-octane
145543-83-5 Downstream products
-
155166-89-5

3,3″-dioctyl-2,2′:5′,2″-trithiophene
-
946535-46-2
3',4''-dioctyl-5'''-(diphenylamino)-2,2':5',2'':5'',2'''-quaterthiophene-5-carboxaldehyde
-
946535-50-8
3'',4'''-dioctyl-5'''''-(diphenylamino)-2,2':5',2'':5'',2''':5''',2'''':5'''',2'''''-sexithiophene-5-carboxaldehyde
-
1334531-08-6
methyl 4-(3-octylthiophen-2-yl)benzoate
Relevant Products
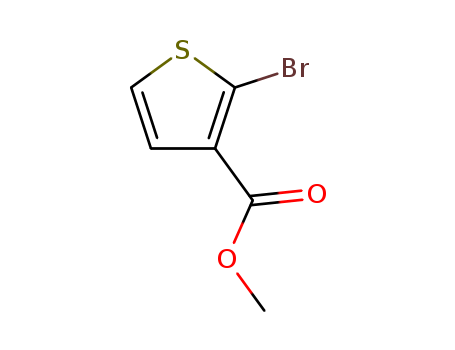
-
Methyl 2-bromothiophene-3-carboxylate
CAS:76360-43-5
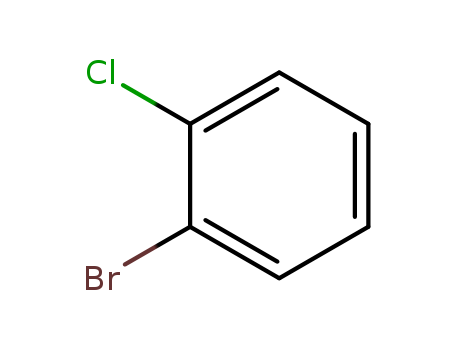
-
2-Bromochlorobenzene
CAS:694-80-4
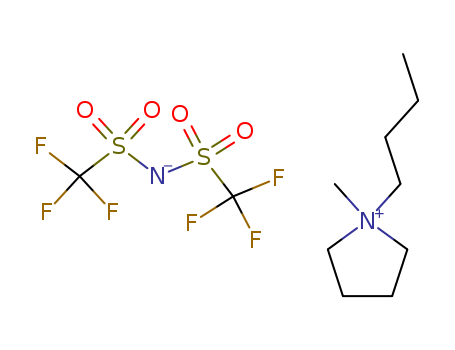
-
1-Butyl-1-methylpyrrolidinium Bis(trifluoromethanesulfonyl)imide
CAS:223437-11-4