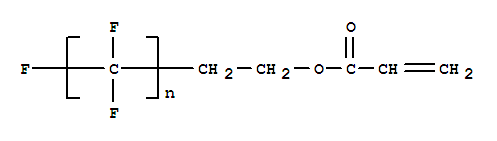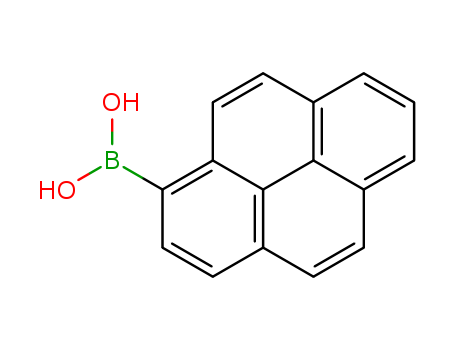
164461-18-1
- Product Name:1-Pyrenylboronic acid
- Molecular Formula:C16H11BO2
- Purity:99%
- Molecular Weight:246.073
Product Details;
CasNo: 164461-18-1
Molecular Formula: C16H11BO2
factory and supplier 164461-18-1 1-Pyrenylboronic acid in stock
- Molecular Formula:C16H11BO2
- Molecular Weight:246.073
- Vapor Pressure:3.36E-11mmHg at 25°C
- Melting Point:247-251 °C(lit.)
- Refractive Index:1.804
- Boiling Point:509.4 °C at 760 mmHg
- PKA:8.53±0.30(Predicted)
- Flash Point:261.9 °C
- PSA:40.46000
- Density:1.35 g/cm3
- LogP:2.26380
1-Pyrenylboronic acid(Cas 164461-18-1) Usage
InChI:InChI=1/C16H11BO2/c18-17(19)14-9-7-12-5-4-10-2-1-3-11-6-8-13(14)16(12)15(10)11/h1-9,18-19H
164461-18-1 Relevant articles
Electron injection into DNA: Synthesis and spectroscopic properties of pyrenyl-modified oligonucleotides
Amann, Nicole,Pandurski, Evgeni,Fiebig, Torsten,Wagenknecht, Hans-Achim
, p. 4877 - 4883 (2002)
The nucleoside 5-(1-pyrenyl)-2′-deoxyuri...
Fast thermal evaporation in purification of 1,4-Di(pyren-1-ly)benzene
Hsu, Chung-Yi,Lin, Hung-Yin,Yan, Xuan-You,Huang, Tsung-Syun,Su, Yan-Kuin,Whang, Thou-Jen
, p. 289 - 296 (2012)
This work presents a fast purification s...
Pyrene boronic acid cyclic ester: A new fast self-recovering mechanoluminescent material at room temperature
Wang, Taisheng,Zhang, Na,Zhang, Ke,Dai, Jingwen,Bai, Wei,Bai, Ruke
, p. 9679 - 9682 (2016)
Two pyrene boronic acid cyclic esters, P...
Tricolor mechanochromic luminescence of an organic two-component dye visualization of a crystalline state and two amorphous states
Ito, Suguru,Katada, Genki,Taguchi, Tomohiro,Kawamura, Izuru,Ubukata, Takashi,Asami, Masatoshi
, p. 53 - 59 (2019)
Solid-state emissive dyes with mechanoch...
Synthesis, in vitro metabolism, cell transformation, mutagenicity, and DNA adduction of dibenzo[c,mno] chrysene
Desai, Dhimant,Sharma, Arun K.,Lin, Jyh-Ming,Krzeminski, Jacek,Pimentel, Maria,El-Bayoumy, Karam,Nesnow, Stephen,Amin, Shantu
, p. 964 - 971 (2002)
Polycyclic aromatic hydrocarbons (PAHs) ...
Excited state processes in ruthenium(II)/pyrenyl complexes displaying extended lifetimes
Tyson, Daniel S.,Henbest, Kevin B.,Bialecki, Jason,Castellano, Felix N.
, p. 8154 - 8161 (2001)
The synthesis and photophysical properti...
Synthesis and luminescent properties of new blue polymer light-emitting diodes material, poly(9-(3-vinyl-phenyl)-pyrene)
Jo, Minjin,Yang, Garam,Lee, Hayoon,Lee, Jaehyun,Jung, Hyocheol,Park, Jongwook
, p. 5669 - 5672 (2017)
Polymer light-emitting diodes (PLEDs) ha...
Tunable mechanochromic luminescence of 2-alkyl-4-(pyren-1-yl)thiophenes: Controlling the self-recovering properties and the range of chromism
Ikeya, Minako,Katada, Genki,Ito, Suguru
, p. 12296 - 12299 (2019)
Controlling the behavior of mechanochrom...
A spirobifluorene derivative as a single-emitting component for a highly efficient white organic electroluminescent device
Wen, Hsin-Yi,Chao, Chun-Ming,Chang, Mei-Ying,Hsieh, Chiung-Wen,Hsieh, Shu-Chen
, p. 1288 - 1295 (2013)
We describe how the morphology, photolum...
Unpredicted Concentration-Dependent Sensory Properties of Pyrene-Containing NBN-Doped Polycyclic Aromatic Hydrocarbons
Bao, Hongli,Chen, Qinghua,Li, Tao,Qian, Qingrong,Sun, Xiao-Li,Wan, Wen-Ming,Xiao, Hang
, (2022/01/10)
Pyrene molecules containing NBN-doped po...
Naphtho-fluorene carbazole compound and application thereof
-
Paragraph 0061; 0067; 0068, (2018/03/26)
The invention belongs to the field of or...
164461-18-1 Process route
-
-
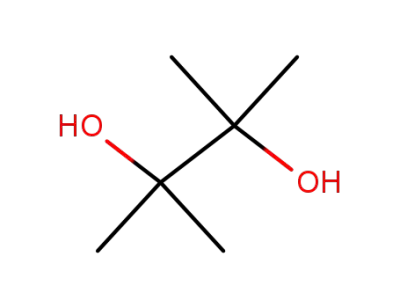
76-09-5
2,3-dimethyl-2,3-butane diol

-
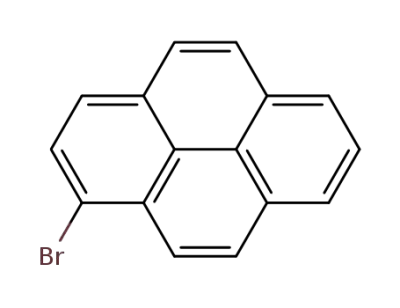
-
1714-29-0
1-bromopyrene

-
-
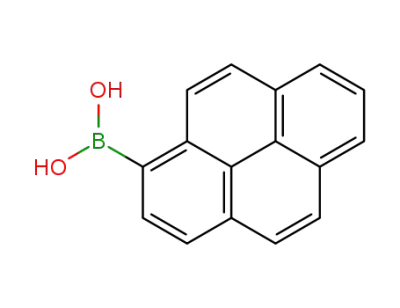
164461-18-1
1-pyrenylboronic acid

-
-
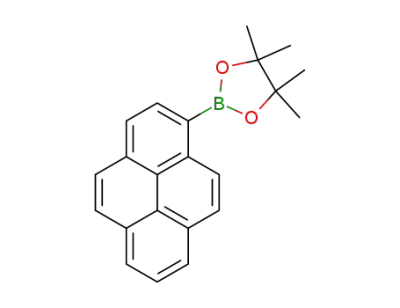
349666-24-6
1-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)pyrene
| Conditions | Yield |
|---|---|
|
1-bromopyrene;
With
n-butyllithium;
In
tetrahydrofuran; hexane;
at -78 ℃;
for 0.5h;
With
Triisopropyl borate;
In
tetrahydrofuran; hexane;
at -78 - 20 ℃;
for 0.5h;
2,3-dimethyl-2,3-butane diol;
In
diethyl ether; hexane;
at 40 ℃;
for 0.75h;
|
85% |
-
-
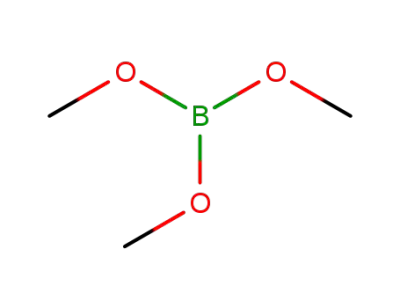
121-43-7,63156-11-6
Trimethyl borate

-
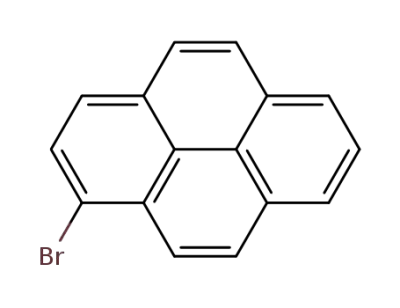
-
1714-29-0
1-bromopyrene

-

-
164461-18-1
1-pyrenylboronic acid
| Conditions | Yield |
|---|---|
|
1-bromopyrene;
With
n-butyllithium;
In
tetrahydrofuran; hexane;
at -78 ℃;
for 1h;
Trimethyl borate;
In
tetrahydrofuran; hexane;
at -78 - 20 ℃;
for 2h;
|
74% |
|
With
n-butyllithium;
In
tetrahydrofuran; hexane;
at -78 - 20 ℃;
for 3h;
|
74% |
|
1-bromopyrene;
With
n-butyllithium;
In
tetrahydrofuran; hexane;
at -78 - 0 ℃;
for 1h;
Inert atmosphere;
Trimethyl borate;
In
tetrahydrofuran; hexane;
at -78 - 20 ℃;
for 24h;
With
hydrogenchloride;
In
tetrahydrofuran; hexane;
for 1h;
|
70% |
|
With
hydrogenchloride; n-butyllithium;
In
diethyl ether; hexane;
BuLi soln. (hexane) addn. to org. compd. soln. (Et2O) at 0°C, mixt. addn. to B-compd. soln. (Et2O) at -78°C over 30 min, stirring 3 h at -50 to -70°C and 60 h at room temp., 2 M HCl addn., stirring 2 h, org. phase sepn.; org. phase washing (water), drying (MgSO4), treating with charcoal, ethereal soln. concn. to dryness, residue vac. drying at 50°C;
|
61% |
|
1-bromopyrene;
With
n-butyllithium;
In
diethyl ether;
Trimethyl borate;
|
164461-18-1 Upstream products
-
1714-29-0
1-bromopyrene
-
76-09-5

2,3-dimethyl-2,3-butane diol
-
121-43-7

Trimethyl borate
-
1033995-63-9
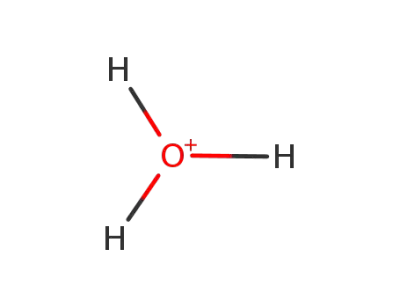
oxonium
164461-18-1 Downstream products
-
448287-48-7
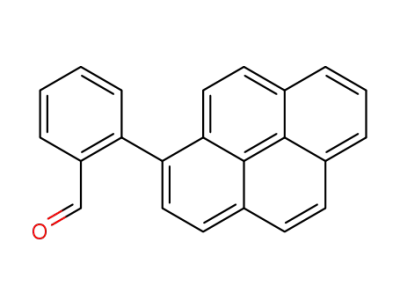
2-(1-Pyrenyl)benzaldehyde
-
448287-51-2
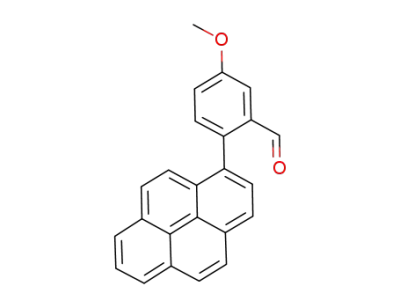
1-(2-formyl-4-methoxyphenyl)pyrene
-
918654-99-6
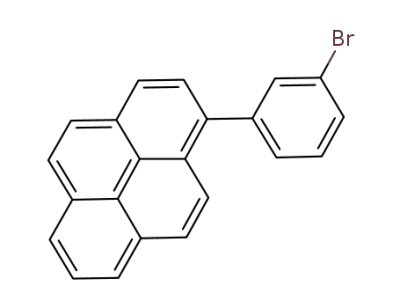
1-(3-bromophenyl)pyrene
-
919791-90-5
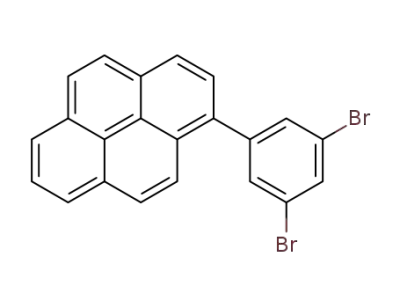
1-(3,5-dibromophenyl)pyrene
Relevant Products
-
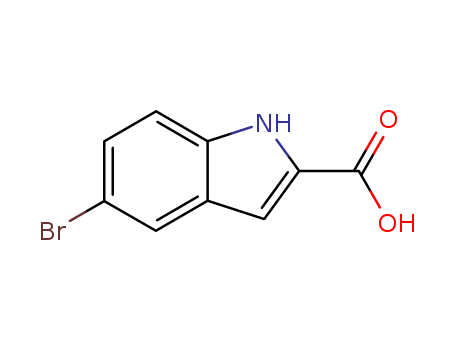
5-Bromoindole-2-carboxylic acid
CAS:7254-19-5
-
Perfluoroalkylethyl acrylate
CAS:65605-70-1
-
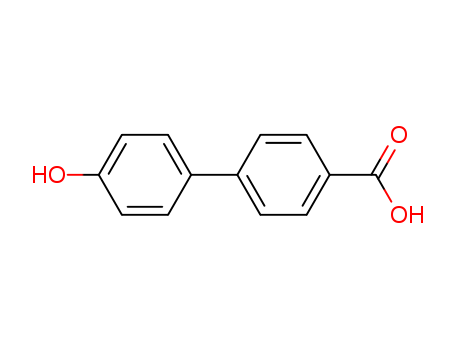
4'-Hydroxy-4-biphenylcarboxylic acid
CAS:58574-03-1