1486-28-8
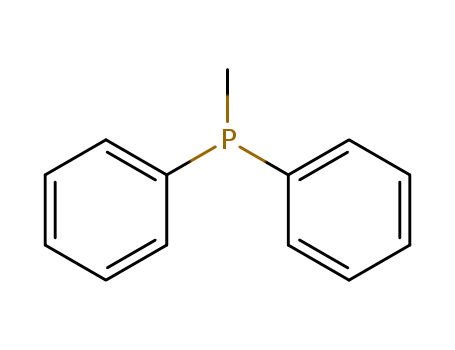
- Product Name:Diphenylphosphinomethane
- Molecular Formula:C13H13P
- Purity:99%
- Molecular Weight:200.22
Product Details;
CasNo: 1486-28-8
Molecular Formula: C13H13P
Appearance: Colorless to light yellow liquid
factory and supplier 1486-28-8 Diphenylphosphinomethane in stock
- Molecular Formula:C13H13P
- Molecular Weight:200.22
- Appearance/Colour:Colorless to light yellow liquid
- Vapor Pressure:0.00127mmHg at 25°C
- Melting Point:117-118 °C
- Refractive Index:n20/D 1.625(lit.)
- Boiling Point:308.1 °C at 760 mmHg
- Flash Point:128.4 °C
- PSA:13.59000
- Density:1.076 g/mL at 25 °C(lit.)
- LogP:2.74910
Methyldiphenylphosphine(Cas 1486-28-8) Usage
|
General Description |
Methyldiphenylphosphine (PPh2Me) is a monodentate phosphine ligand used in the synthesis of cycloplatinated(II) complexes, where it replaces dimethyl sulfoxide (DMSO) in [PtMe(Vpy)(DMSO)] to form [PtMe(Vpy)(PPh2Me)]. This complex exhibits intense phosphorescence emission in both solid state and solution, attributed to a mixed 3ILCT/3MLCT excited state, and undergoes oxidative addition reactions with alkyl halides via an SN2 mechanism, demonstrating its utility in optoelectronic and catalytic applications. |
InChI:InChI=1/C13H13P/c1-14(12-8-4-2-5-9-12)13-10-6-3-7-11-13/h2-11H,1H3
1486-28-8 Relevant articles
Br?nsted Acid Promoted Reduction of Tertiary Phosphine Oxides
Krachko, Tetiana,Lyaskovskyy, Volodymyr,Lutz, Martin,Lammertsma, Koop,Slootweg, J. Chris
, p. 916 - 921 (2017)
Recently, Br?nsted acids, such as phosph...
-
Seyferth,Burlitch
, p. 2463 (1963)
-
Ethylene Tetramerization Catalysis: Effects of Aluminum-Induced Isomerization of PNP to PPN Ligands
Lifschitz, Alejo M.,Hirscher, Nathanael A.,Lee, Heui Beom,Buss, Joshua A.,Agapie, Theodor
, p. 1640 - 1648 (2017)
Diphosphinoamines (PNP) are commonly use...
Palladium-Catalyzed Cleavage of P-C Bonds In Quaternary Phosphonium Salts and Its Applications to Organic Synthesis
Sakamoto, Masato,Shimizu, Isao,Yamamoto, Akio
, p. 1101 - 1102 (1995)
Phosphonium salts, PPh4I and PMePh3I, ox...
-
Eyles,Trippett
, p. 67,69 (1966)
-
Dimethylsulfonium methylide in methylation of silylphosphines
Veits,Chuchuryukin,Neganova
, p. 1790 - 1792 (2002)
Selective monodesilylation of bissilylat...
Alane - A novel way to reduce phosphine oxides
Griffin, Sara,Heath, Lucy,Wyatt, Paul
, p. 4405 - 4406 (1998)
Phosphine oxides may be reduced to phosp...
Preparation and properties of a series of structurally diverse aluminium hydrides supported by β-diketiminate and bis(amide) ligands
Nako, Adi E.,Gates, Sarah J.,White, Andrew J.P.,Crimmin, Mark R.
, p. 15199 - 15206 (2013)
The synthesis of a diverse series of hyd...
A Lewis Base Nucleofugality Parameter, NFB, and Its Application in an Analysis of MIDA-Boronate Hydrolysis Kinetics
García-Domínguez, Andrés,Gonzalez, Jorge A.,Leach, Andrew G.,Lloyd-Jones, Guy C.,Nichol, Gary S.,Taylor, Nicholas P.
supporting information, (2022/01/04)
The kinetics of quinuclidine displacemen...
The Trityl-Cation Mediated Phosphine Oxides Reduction
Landais, Yannick,Laye, Claire,Lusseau, Jonathan,Robert, Frédéric
supporting information, p. 3035 - 3043 (2021/05/10)
Reduction of phosphine oxides into the c...
Reversing Lewis acidity from bismuth to antimony
Balasubramaniam, Selvakumar,Jemmis, Eluvathingal D.,Kumar, Sandeep,Sharma, Deepti,Venugopal, Ajay
supporting information, p. 8889 - 8892 (2021/09/10)
Investigations on the boundaries between...
Reductive conversion of phosphoryl P(O) compounds to trivalent organophosphines R3P
Zhang, Jian-Qiu,Han, Li-Biao
, (2021/02/20)
By introducing trimethylsilyl chloride (...
1486-28-8 Process route
-
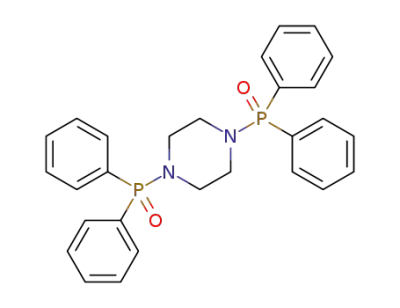
-
piperazine-1,4-diylbis(diphenylphosphine)oxide

-
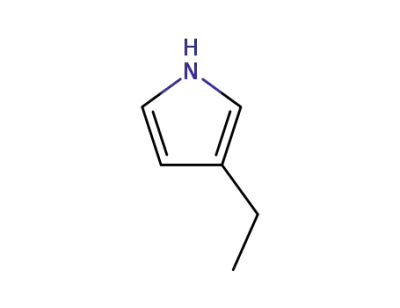
-
1551-16-2
3-ethyl-1H-pyrrole

-
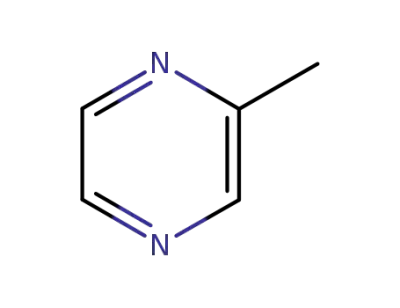
-
109-08-0
2-Methylpyrazine

-
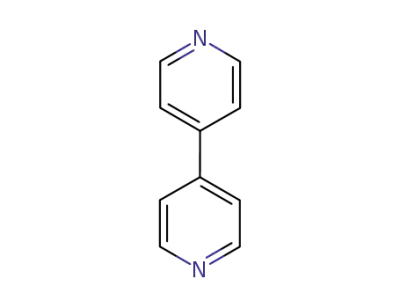
-
553-26-4
4,4'-bipyridine

-
-
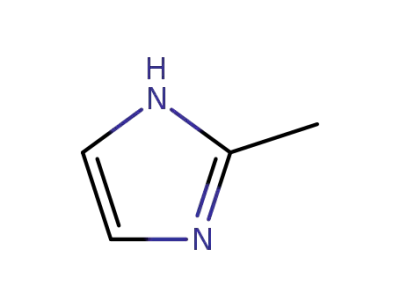
693-98-1
2-methylimidazole

-
-
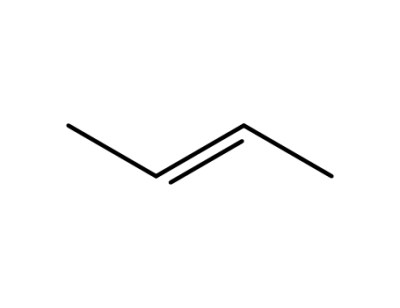
624-64-6
trans-2-Butene

-
-
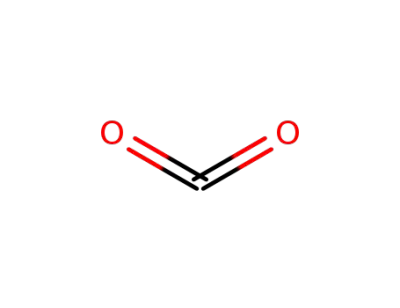
124-38-9,18923-20-1
carbon dioxide

-
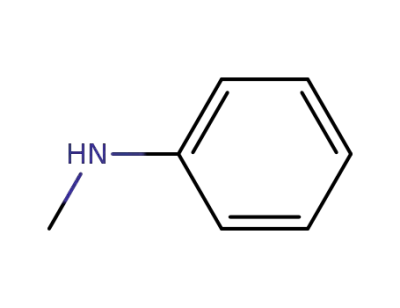
-
100-61-8
N-methylaniline

-
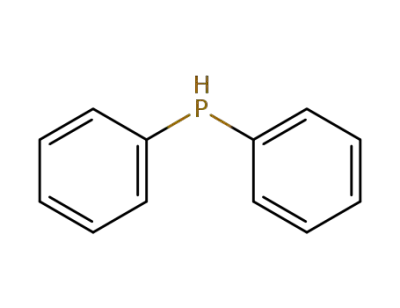
-
829-85-6
diphenylphosphane

-
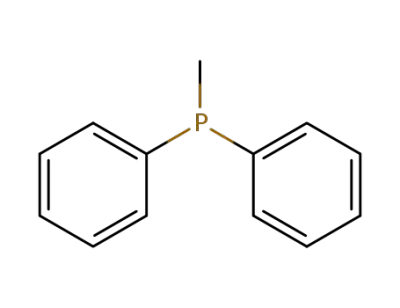
-
1486-28-8
diphenyl(methyl)phosphine

-
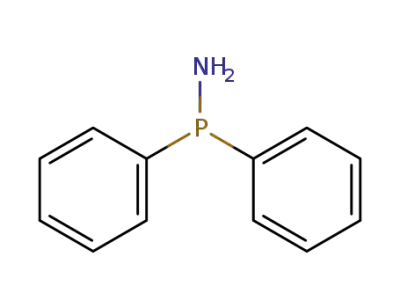
-
17157-61-8
bis(diphenylphosphino)amine
| Conditions | Yield |
|---|---|
|
at 600 ℃;
|
-
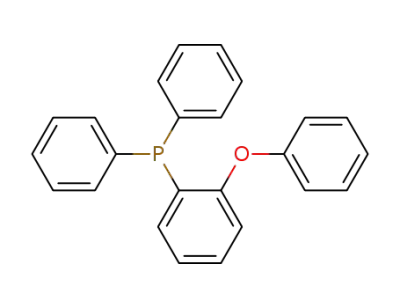
-
183383-68-8
2-phenoxyphenyldiphenylphosphine

-
-
917-54-4
methyllithium

-
-
1225-16-7
10-phenyl-10H-phenoxaphosphinine

-
-
22472-42-0
10-methyl-10H-phenoxaphosphine

-

-
603-35-0
triphenylphosphine

-

-
1486-28-8
diphenyl(methyl)phosphine
| Conditions | Yield |
|---|---|
|
With
water;
Multistep reaction;
|
|
|
Product distribution;
reactions of tertiary phosphine derivatives of diphenyl ether with lithium reagents; possible mechanism;
|
1486-28-8 Upstream products
-
60-29-7
diethyl ether
-
124-41-4
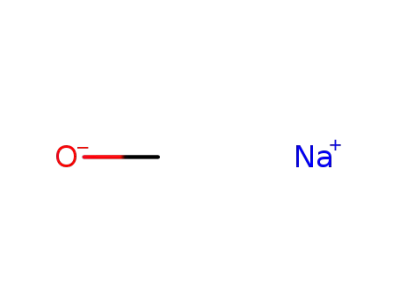
sodium methylate
-
1079-66-9
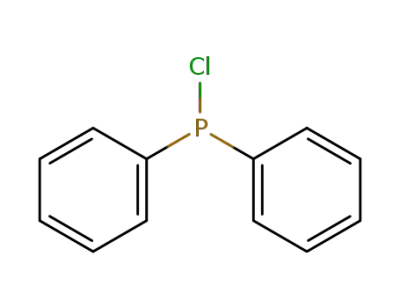
chloro-diphenylphosphine
-
544-97-8
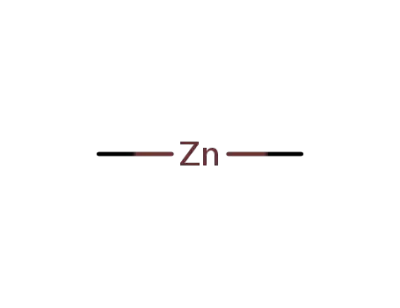
dimethyl zinc(II)
1486-28-8 Downstream products
-
1661-08-1
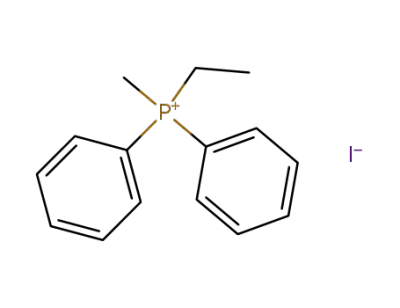
diphenylethylmethylphosphonium iodide
-
106141-00-8
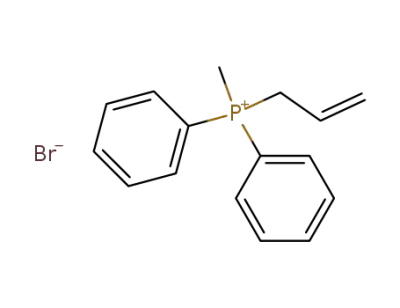
methylallyldiphenylphosphonium bromide
-
1017-88-5
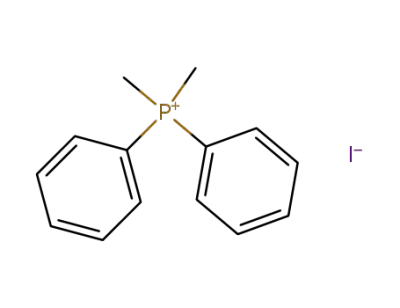
dimethyldiphenylphosphonium iodide
-
2129-89-7
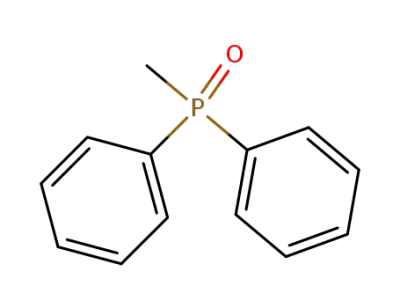
diphenyl(methyl)phosphine oxide
Relevant Products
-
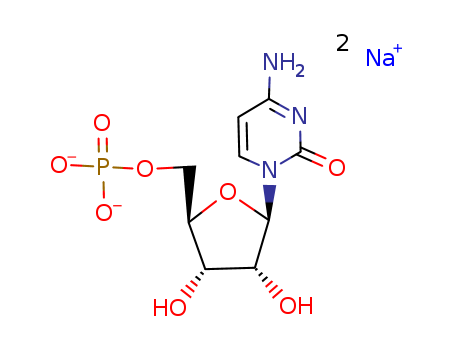
Cytidine 5'-monophosphate disodium salt
CAS:6757-06-8
-
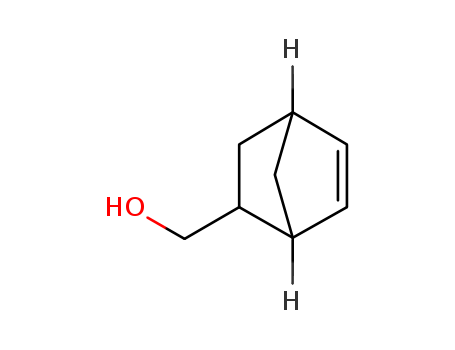
5-Norbornene-2-methanol
CAS:95-12-5
-
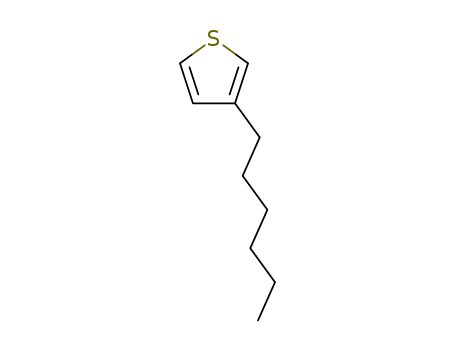
3-Hexylthiophene
CAS:1693-86-3