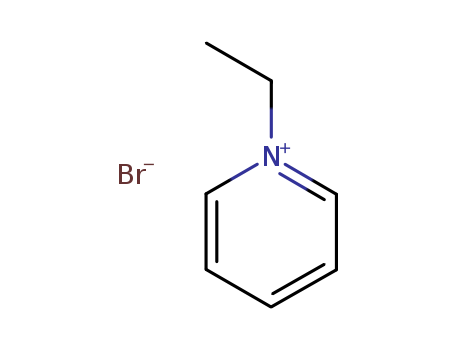
1693-86-3
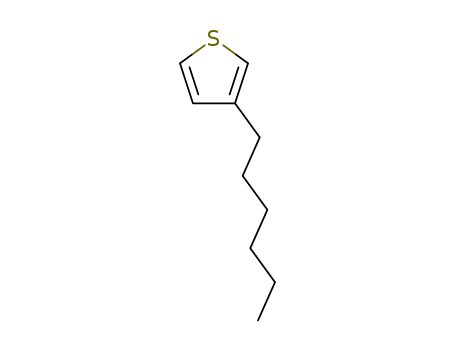
- Product Name:3-Hexylthiophene
- Molecular Formula:C10H16S
- Purity:99%
- Molecular Weight:168.303
Product Details;
CasNo: 1693-86-3
Molecular Formula: C10H16S
Appearance: colorless transparent or light yellow liquid
factory and supplier 1693-86-3 3-Hexylthiophene in stock
- Molecular Formula:C10H16S
- Molecular Weight:168.303
- Appearance/Colour:colorless transparent or light yellow liquid
- Vapor Pressure:0.128mmHg at 25°C
- Melting Point:-39.15°C (estimate)
- Refractive Index:n20/D 1.496(lit.)
- Boiling Point:225.6 °C at 760 mmHg
- Flash Point:64.2 °C
- PSA:28.24000
- Density:0.946 g/cm3
- LogP:3.87090
3-Hexylthiophene(Cas 1693-86-3) Usage
|
Preparation |
The 2,5-dibromo-3-dodecylthiophene was dissolved in tetrahydromyran, added with methylmagnesium bromide, preheated and added with catalyst for reaction reaction was injected into alcohol solvent, and hexane was added to remove the copolymer, and then the mixture was soxhlet filtered using chloroform, and the chloroform layer was evaporated and concentrated to obtain a purple membrane; the purple membrane was vacuum filtered to obtain the product 3-hexylthiophene. |
|
General Description |
3-Hexylthiophene, a sulfur containing heterocyclic building block, is a thiophene derivative. Poly(3-hexylthiophene) (P3HT) nanofibres have been used for the preparation of organic phototransistors (OPTs). |
InChI:InChI=1/C10H16S/c1-2-3-4-5-6-10-7-8-11-9-10/h7-9H,2-6H2,1H3
1693-86-3 Relevant articles
Synthesis and photovoltaic properties of low-bandgap 4,7-dithien-2-yl-2,1, 3-benzothiadiazole-based poly(heteroarylenevinylene)s
Wen, Shanpeng,Pei, Jianing,Li, Pengfei,Zhou, Yinhua,Cheng, Weidong,Dong, Qingfeng,Li, Zaifang,Tian, Wenjing
, p. 2715 - 2724 (2011)
Three novel low-bandgap copolymers conta...
Physical, mechanical, and conductivity properties of poly(3-hexylthiophene) -montmorillonite clay nanocomposites produced by the solvent casting method
Kuila, Biplab K.,Nandi, Arun K.
, p. 8577 - 8584 (2004)
Polymer nanocomposites (PNCs) of poly(3-...
Regiospecific Synthesis of 3-Alkylfuranes and 3-Alkylthiophenes via Organoboranes
Akimoto, Itaru,Sano, Masahiro,Suzuki, Akira
, p. 1587 - 1588 (1981)
The reaction of bromide or iodide with a...
Redox-Divergent Construction of (Dihydro)thiophenes with DMSO
Chen, Qing-An,He, Gu-Cheng,Hu, Yan-Cheng,Ji, Ding-Wei,Liu, Heng,Zhang, Xiang-Xin,Zhao, Chao-Yang
supporting information, p. 24284 - 24291 (2021/10/08)
Thiophene-based rings are one of the mos...
1,2,3-Triazolyl functionalized thiophene, carbazole and fluorene based A-: Alt -B type π-conjugated copolymers for the sensitive and selective detection of aqueous and vapor phase nitroaromatics (NACs)
Giri, Dipanjan,Patra, Sanjib K.
supporting information, p. 14469 - 14480 (2020/11/09)
A series of highly emissive π-conjugated...
Efficient Pd-Catalyzed Direct Coupling of Aryl Chlorides with Alkyllithium Reagents
Dilchert, Katharina,Gessner, Viktoria H.,Gro?johann, Angela,Rodstein, Ilja,Scherpf, Thorsten,Steinert, Henning,Tappen, Jens
supporting information, p. 20596 - 20603 (2020/09/09)
Organolithium compounds are amongst the ...
Conductive triethylene glycol monomethyl ether substituted polythiophenes with high stability in the doped state
Dissanayake, Dushanthi S.,Gunathilake, Samodha S.,Udamulle Gedara, Chinthaka M.,Du, Jia,Yoo, Sang Ha,Lee, Youngmin,Wang, Qing,Gomez, Enrique D.,Biewer, Michael C.,Stefan, Mihaela C.
, p. 1079 - 1086 (2019/03/21)
Synthesis of two conducting polymers con...
1693-86-3 Process route
-
-
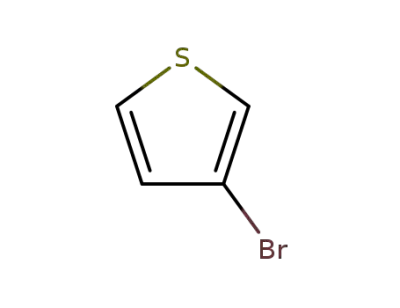
872-31-1
3-Bromothiophene

-
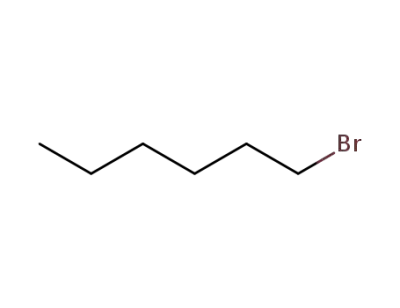
-
111-25-1
1-bromo-hexane

-
-
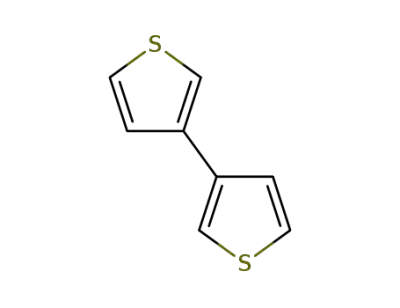
3172-56-3
3,3'-bithiophene

-

-
740804-46-0
3-(1-methylpentyl)thiophene

-

-
1693-86-3
3-hexylthiophene
| Conditions | Yield |
|---|---|
|
1-bromo-hexane; n-hexylmagnesium bromide;
With
magnesium;
In
2-methyltetrahydrofuran;
at 60 - 85 ℃;
for 4.5h;
3-Bromothiophene;
1,3-bis[(diphenylphosphino)propane]dichloronickel(II);
In
2-methyltetrahydrofuran;
at 15 - 20 ℃;
for 20h;
With
hydrogenchloride; water;
In
2-methyltetrahydrofuran;
Product distribution / selectivity;
|
97.5 - 98.88 %Chromat. 0.37 - 0.45 %Chromat. 0.15 - 0.49 %Chromat. |
-

-
872-31-1
3-Bromothiophene

-
-
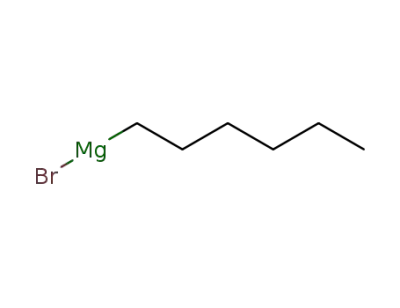
3761-92-0
n-hexylmagnesium bromide

-

-
3172-56-3
3,3'-bithiophene

-

-
1693-86-3
3-hexylthiophene
| Conditions | Yield |
|---|---|
|
bis(triphenylphosphine)nickel(II) chloride;
In
tetrahydrofuran;
at 0 - 60 ℃;
for 2.5h;
Product distribution / selectivity;
|
36.2 - 82.7 %Chromat. 6.4 - 27.1 %Chromat. |
|
bis-triphenylphosphine-palladium(II) chloride;
In
diethyl ether;
at 20 ℃;
for 2.5h;
Product distribution / selectivity;
|
4.0 - 4.5 %Chromat. 0.2 - 0.5 %Chromat. |
|
bis(triphenylphosphine)nickel(II) chloride;
In
diethyl ether;
at 20 ℃;
for 1h;
Product distribution / selectivity;
|
1.7 - 3.1 %Chromat. 0.5 - 6.3 %Chromat. |
|
In
diethyl ether;
at 20 ℃;
for 1h;
Conversion of starting material;
|
0.5 - 1.1 %Chromat. 0.2 - 0.4 %Chromat. |
1693-86-3 Upstream products
-
872-31-1

3-Bromothiophene
-
111-25-1
1-bromo-hexane
-
1188-92-7
Trihexylboran; Tri-n-hexylbor
-
69249-61-2
3-hexyl-2-bromothiophene
1693-86-3 Downstream products
-
69249-61-2

3-hexyl-2-bromothiophene
-
210705-84-3
2-bromo-4-n-hexylthiophene
-
116971-11-0
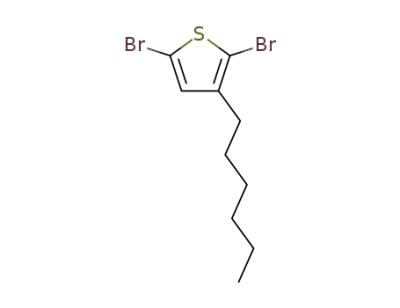
2,5-dibromo-3-hexylthiophene
-
748763-44-2
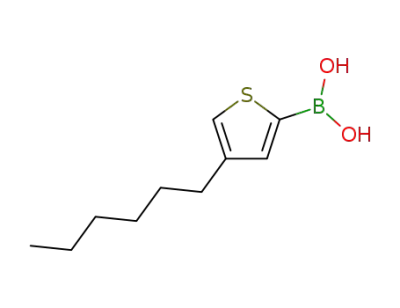
(4-hexylthiophen-2-yl)boronic acid
Relevant Products
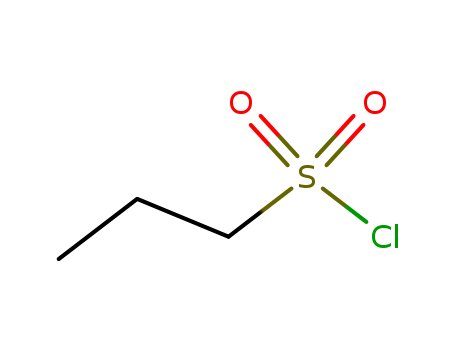
-
Propanesulphonyl chloride
CAS:10147-36-1
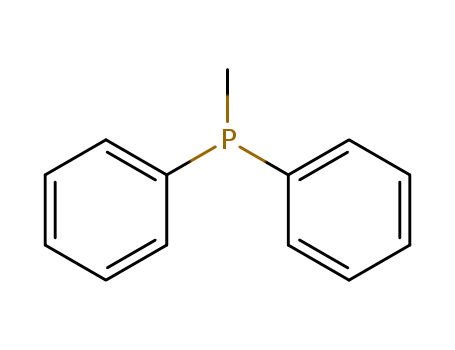
-
Diphenylphosphinomethane
CAS:1486-28-8
-
1-Ethylpyridinium Bromide
CAS:1906-79-2