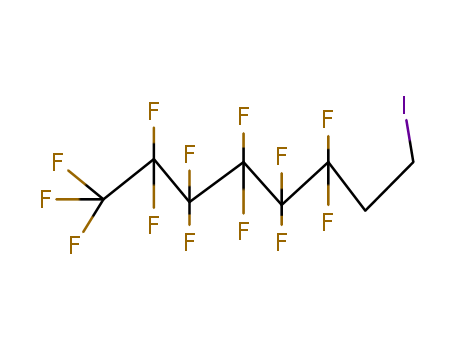
2043-55-2
- Product Name:1H,1H,2H,2H-Perfluorohexyl iodide
- Molecular Formula:C6H4F9I
- Purity:99%
- Molecular Weight:373.988
Product Details;
CasNo: 2043-55-2
Molecular Formula: C6H4F9I
factory and supplier 2043-55-2 1H,1H,2H,2H-Perfluorohexyl iodide in stock
- Molecular Formula:C6H4F9I
- Molecular Weight:373.988
- Vapor Pressure:4.88mmHg at 25°C
- Melting Point:-25°C
- Refractive Index:n20/D 1.370
- Boiling Point:150.492 °C at 760 mmHg
- Flash Point:58.517 °C
- PSA:0.00000
- Density:1.912 g/cm3
- LogP:4.27970
1H,1H,2H,2H-Perfluorohexyl iodide(Cas 2043-55-2) Usage
InChI:InChI=1/C6H4F9I/c7-3(8,1-2-16)4(9,10)5(11,12)6(13,14)15/h1-2H2
2043-55-2 Relevant articles
Controlled step-wise telomerization of vinylidene fluoride, hexafluoropropene and trifluoroethylene with iodofluorinated transfer agents
Balagué,Améduri,Boutevin,Caporiccio
, p. 253 - 268 (2000)
Highly fluorinated cotelomers having the...
Quinim: A New Ligand Scaffold Enables Nickel-Catalyzed Enantioselective Synthesis of α-Alkylated ?-Lactam
Chen, Yifeng,Qu, Jingping,Wu, Xianqing
supporting information, p. 15654 - 15660 (2020/10/18)
Herein, we report a nickel-catalyzed red...
Environmentally friendly preparation method of fluorine-containing acrylate
-
Paragraph 0018; 0021; 0028; 0031, (2019/01/06)
The invention relates to the technical f...
Ethylene-tetrafluoroethylene (ETFE) cotelomer iodides and their transformation to surface protection intermediates
Qiu, Weiming,Raghavanpillai, Anilkumar,Brown, Peter A.,Atkinson, Wayne R.,Vincent, Michael F.,Marshall, William J.
, p. 12 - 23 (2015/03/05)
A new class of fluorotelomers was synthe...
Purification of fluorinated alcohols
-
Page/Page column 4, (2008/06/13)
A process for reducing the level of perf...
2043-55-2 Process route

-
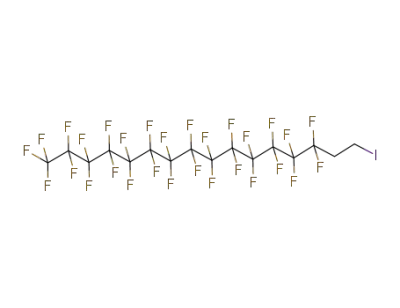
-
65510-55-6
1-iodo-1H,1H,2H,2H-perfluorohexadecane

-
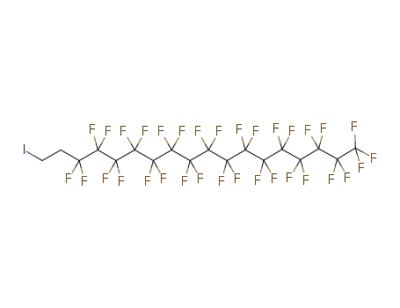
-
1-iodo-1H,1H,2H,2H-perfluorooctadecane

-
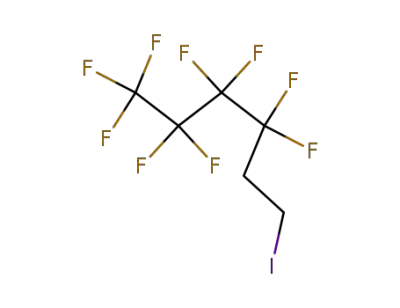
-
2043-55-2,71215-70-8
3,3,4,4,5,5,6,6,6-nonafluorohexyliodide

-
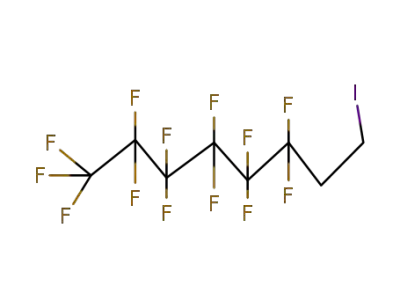
-
2043-57-4,71215-70-8
1,1,1,2,2,3,3,4,4,5,5,6,6-Tridecafluoro-8-iodooctane

-
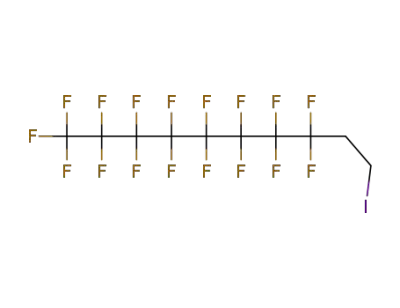
-
2043-53-0,71215-70-8
2-(n-perfluorooctyl)ethyl iodide

-
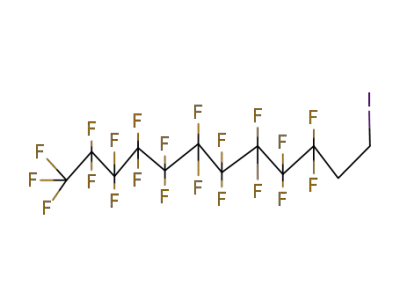
-
2043-54-1,71215-70-8
1-iodo-1H,1H,2H,2H-perfluorododecane

-
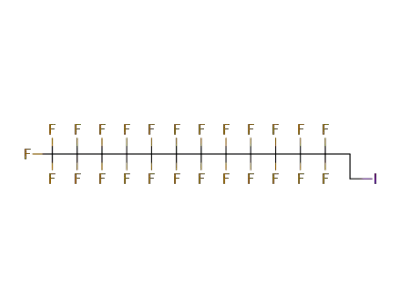
-
30046-31-2
1-iodo-1H,1H,2H,2H-perfluorotetradecane
| Conditions | Yield |
|---|---|
|
Purification / work up;
|
-
-
74-85-1
ethene

-
-
423-39-2
1-iodo-2,2,3,3,4,4,5,5,5-nonafluorobutane

-

-
2043-55-2,71215-70-8
3,3,4,4,5,5,6,6,6-nonafluorohexyliodide
| Conditions | Yield |
|---|---|
|
at 205 ℃;
for 24h;
|
50% |
|
at 200 ℃;
Yield given;
|
|
|
With
Vazo64;
|
|
|
With
2,2'-azobis(isobutyronitrile);
In
isopropyl alcohol;
at 60 - 70 ℃;
for 3.5h;
under 3375.34 Torr;
Solvent;
Temperature;
Pressure;
Large scale;
Green chemistry;
|
85.3 kg |
2043-55-2 Upstream products
-
74-85-1
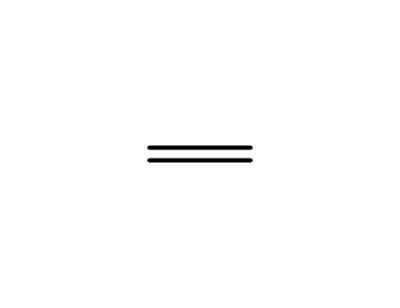
ethene
-
423-39-2

1-iodo-2,2,3,3,4,4,5,5,5-nonafluorobutane
-
38436-14-5
1H,1H,2H,2H-perfluoro-n-hexyl bromide
2043-55-2 Downstream products
-
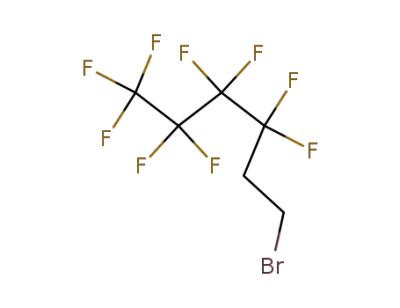
19430-93-4
3,3,4,4,5,5,6,6,6-Nonafluoro-1-hexene
-
80705-13-1
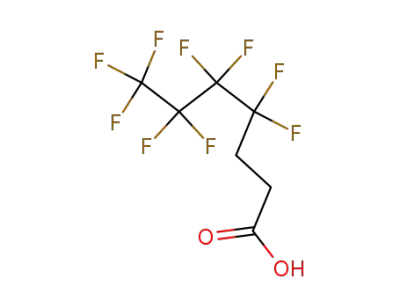
4,4,5,5,6,6,7,7,7-nonafluoroheptanoic acid
-
2043-47-2
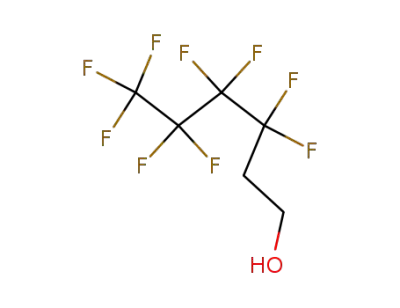
1H,1H,2H,2H-nonafluoro-1-hexanol
-
34451-25-7
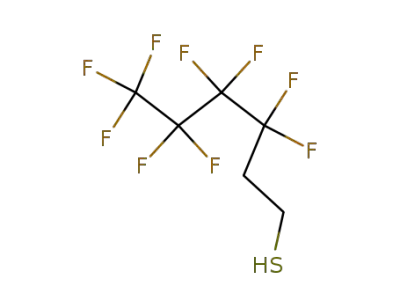
3,3,4,4,5,5,6,6,6-nonafluoro-hexane-1-thiol
Relevant Products
-
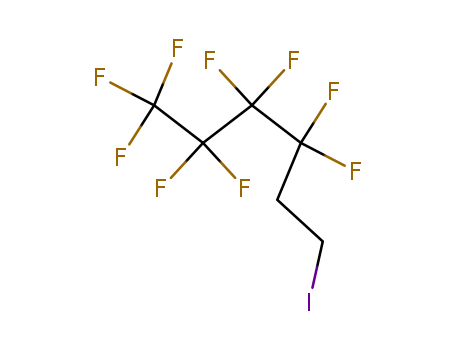
1H,1H,2H,2H-Perfluorooctyl iodide
CAS:2043-57-4
-
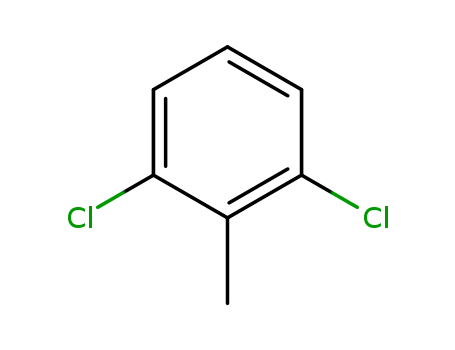
2,6-Dichlorotoluene
CAS:118-69-4