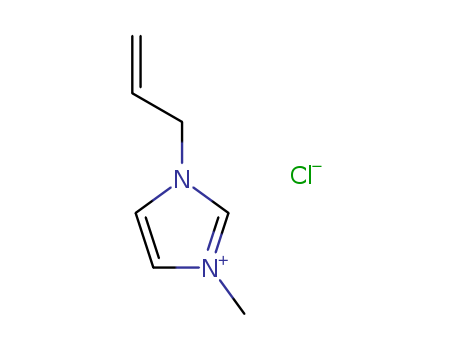
65039-10-3
- Product Name:1-Allyl-3-ButylImidazolium Chloride
- Molecular Formula:C7H11N2.Cl
- Purity:99%
- Molecular Weight:158.631
Product Details;
CasNo: 65039-10-3
Molecular Formula: C7H11N2.Cl
factory and manufacture 65039-10-3 1-Allyl-3-ButylImidazolium Chloride lonic liquid
- Molecular Formula:C7H11N2.Cl
- Molecular Weight:158.631
- Melting Point:55℃
- Refractive Index:1.540 (50℃)
- PSA:8.81000
- LogP:-2.49740
1-ALLYL-3-METHYLIMIDAZOLIUM CHLORIDE(Cas 65039-10-3) Usage
|
General Description |
We are committed to bringing you Greener Alternative Products, which adhere to one or more of The 12 Principles of Greener Chemistry. This product has been enhanced for catalytic efficiency. Click here for more information. |
InChI:InChI=1/C7H11N2.ClH/c1-3-4-9-6-5-8(2)7-9;/h3,5-7H,1,4H2,2H3;1H/q+1;/p-1
65039-10-3 Relevant articles
Separation and recovery of cellulose from Zoysia japonica by 1-allyl-3-methylimidazolium chloride
Li, Wei-Zun,Ju, Mei-Ting,Wang, Yan-Nan,Liu, Le,Jiang, Yang
, p. 228 - 235 (2013)
We investigated the use of ionic liquid ...
Catalytic conversion of fructose and sucrose to 5-hydroxymethylfurfural using simple ionic liquid/DMF binary reaction media
Shi, Jincai,Yang, Yan,Wang, Ningning,Song, Zhanxin,Gao, Haiyan,Xia, Yongmei,Li, Wei,Wang, Haijun
, p. 89 - 92 (2013)
The production of 5-hydroxymethylfurfura...
Task-specific ionic liquid for the depolymerisation of starch-based industrial waste into high reducing sugars
Hernoux-Villière, Audrey,Lévêque, Jean-Marc,K?rkk?inen, Johanna,Papaiconomou, Nicolas,Lajunen, Marja,Lassi, Ulla
, p. 11 - 17 (2014)
Development of a simple route for the ca...
Successful application of an ionic liquid carrying the fluoride counter-ion in biomacromolecular chemistry: Microwave-Assisted acylation of cellulose in the presence of 1-allyl-3-methylimidazolium fluoride/DMSO mixtures
Casarano, Romeu,El Seoud, Omar A.
, p. 191 - 202 (2013)
The use of ionic liquids with fluoride a...
Pretreatment of fibre sludge in ionic liquids followed by enzyme and acid catalysed hydrolysis
Holm, Jana,Lassi, Ulla,Romar, Henrik,Lahti, Riikka,K?rkk?inen, Johanna,Lajunen, Marja
, p. 11 - 15 (2012)
Pretreatment of fibre sludge in ionic li...
Functional ionic liquids for hydrolysis of lignocellulose
Hu, Xiaomei,Xiao, Yibo,Niu, Kun,Zhao, Yang,Zhang, Bixian,Hu, Baozhong
, p. 172 - 176 (2013)
An efficient system for hydrolysis of li...
Dissolution of cellulose from AFEX-pretreated Zoysia japonica in AMIMCl with ultrasonic vibration
Liu, Le,Ju, Meiting,Li, Weizun,Hou, Qidong
, p. 412 - 420 (2013)
In this study, 1-allyl-3-methylimidazoli...
Dissolution of cellulose in 1-allyl-3-methylimizodalium carboxylates at room temperature: A structure-property relationship study
Zhang, Yajuan,Xu, Airong,Lu, Benlian,Li, Zhiyong,Wang, Jianji
, p. 666 - 672 (2015)
The development of highly efficient cell...
Synthetic, spectroscopic and structural behavior of unsaturated functionalized N-heterocyclic carbene complexes of group 11
González-Abrego, Daniel Omar,Zuno-Cruz, Francisco J.,Carpio-Granillo, Mariana,Andrade-López, Noemí,Cruz-Borbolla, Julián,Martínez-Macias, Claudia,Mendoza-Espinosa, Daniel,Rosales-Hoz, María J.,Leyva, Marco A.,Torres-Lubián, José R.,López-Jiménez, Jorge A.,Jancik, Vojtech,Sánchez-Cabrera, Gloria
, p. 97 - 111 (2017)
A series of unsaturated functionalized s...
Investigations about dissolution of cellulose in the 1-allyl-3- alkylimidazolium chloride ionic liquids
Liu, De-Tao,Xia, Kun-Feng,Cai, Wei-Hua,Yang, Ren-Dang,Wang, Li-Qin,Wang, Bin
, p. 1058 - 1064 (2012)
In this work, the 1-allyl-3-alkylimidazo...
Dissolution of feather keratin in ionic liquids
Idris, Azila,Vijayaraghavan,Rana, Usman Ali,Fredericks, Dale,Patti,MacFarlane
, p. 525 - 534 (2013)
Keratin from various livestock industrie...
Synthesis and characterization of nitrogen-based ionic liquids bearing allyl groups and examples of their application
Zajac, Adrian,Szpecht, Andrea,Szymanska, Anna,Zielinski, Dawid,Stolarska, Olga,Smiglak, Marcin,Maciejewski, Hieronim
, p. 12274 - 12288 (2020/07/30)
The syntheses and full spectral (NMR, MS...
Understanding the efficiency of ionic liquids-DMSO as solvents for carbohydrates: use of solvatochromic- And related physicochemical properties
Bioni, Thaís A.,de Oliveira, Mayara L.,Dignani, Marcella T.,El Seoud, Omar A.
, p. 14906 - 14914 (2020/09/23)
The physical dissolution of carbohydrate...
Olefin-tethered organoruthenium carbene complexes: Synthesis, X-ray structure and catalytic insights on hydrogenation of esters
Nirmala, Muthukumaran,Murugan, Kaliyappan,Vijayapritha, Subbarayan,Viswanathamurthi, Periasamy,Bertani, Roberta,Malecki, Jan Grzegorz
, p. 55 - 62 (2018/10/25)
A series of Ru(II) complexes encompassin...
Direct catalytic conversion of glucose and cellulose
Li, Zhenhuan,Su, Kunmei,Ren, Jun,Yang, Dongjiang,Cheng, Bowen,Kim, Chan Kyung,Yao, Xiangdong
supporting information, p. 863 - 872 (2018/03/05)
Biomass product 5-hydroxymethylfurfural ...
65039-10-3 Process route
-
-
616-47-7
1-methyl-1H-imidazole

-
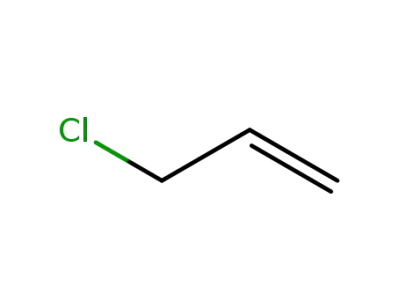
-
107-05-1
3-chloroprop-1-ene

-
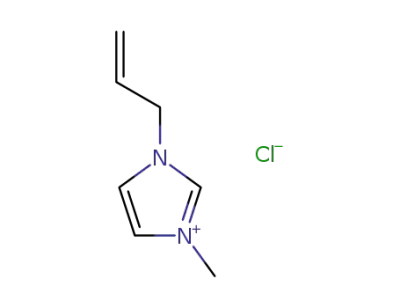
-
65039-10-3
1-allyl-3-methylimidazolium chloride
| Conditions | Yield |
|---|---|
|
In
neat liquid;
at 55 ℃;
for 18h;
Reflux;
Inert atmosphere;
|
100% |
|
at 55 ℃;
for 18h;
Inert atmosphere;
|
100% |
|
at 0 ℃;
for 24h;
Reflux;
Inert atmosphere;
|
98% |
|
In
acetonitrile;
at 60 ℃;
for 96h;
|
98% |
|
In
neat (no solvent);
at 50 - 60 ℃;
for 24h;
Inert atmosphere;
|
97% |
|
at 180 ℃;
for 0.00694444h;
microwave irradiation;
|
90% |
|
In
neat (no solvent);
at 70 ℃;
for 1.25h;
Irradiation;
|
90% |
|
In
toluene;
at 0 - 110 ℃;
for 24h;
|
90% |
|
In
acetonitrile;
at 70 ℃;
for 2h;
Microwave irradiation;
|
90% |
|
In
tetrahydrofuran;
for 15h;
Reflux;
Inert atmosphere;
Schlenk technique;
|
84% |
|
Heating;
|
82% |
|
In
toluene;
at 105 ℃;
for 72h;
|
76% |
|
In
acetonitrile;
at 85 ℃;
for 6h;
under 7600.51 Torr;
Inert atmosphere;
|
|
|
at 60 ℃;
for 8h;
|
|
|
at 55 ℃;
for 10h;
Inert atmosphere;
|
|
|
at 79.84 ℃;
for 8h;
|
|
|
at 40 ℃;
for 10h;
Inert atmosphere;
Cooling with ice;
|
|
|
In
neat (no solvent);
at 60 ℃;
for 7h;
|
|
|
at 55 ℃;
for 10h;
Inert atmosphere;
|
|
|
at 55 ℃;
for 18h;
Inert atmosphere;
Cooling with ice;
|
|
|
at 55 ℃;
for 8h;
|
|
|
at 55 ℃;
for 18h;
|
|
|
at 55 ℃;
for 8h;
|
|
|
at 55 ℃;
for 8h;
Inert atmosphere;
|
|
|
In
toluene;
at 90 ℃;
Inert atmosphere;
|
315 g |
|
at 60 ℃;
for 0.166667h;
Microwave irradiation;
|
96 %Spectr. |
|
at 50 ℃;
for 12h;
|
142.5 g |
|
at 55 ℃;
Inert atmosphere;
|
|
|
at 25 ℃;
for 24h;
|
-

-
616-47-7
1-methyl-1H-imidazole

-
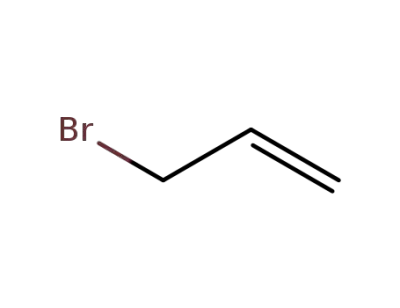
-
106-95-6
allyl bromide

-

-
65039-10-3
1-allyl-3-methylimidazolium chloride
| Conditions | Yield |
|---|---|
|
In
acetonitrile;
Schlenk technique;
|
91% |
|
In
diethyl ether;
|
65039-10-3 Upstream products
-
616-47-7

1-methyl-1H-imidazole
-
107-05-1

3-chloroprop-1-ene
-
106-95-6
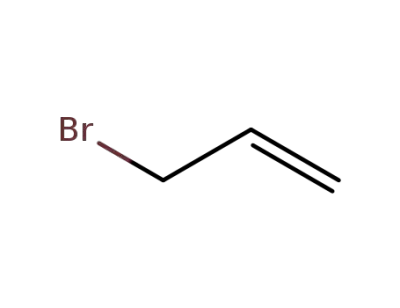
allyl bromide
65039-10-3 Downstream products
-
616-47-7

1-methyl-1H-imidazole
-
70529-85-0
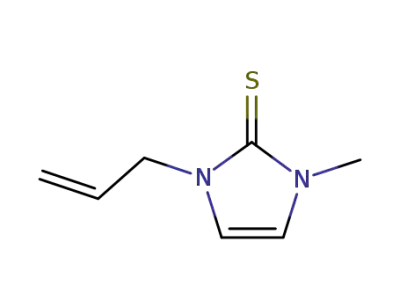
1-methyl-3-(2-propenyl)-2(3H)-imidazolethione
-
655249-87-9
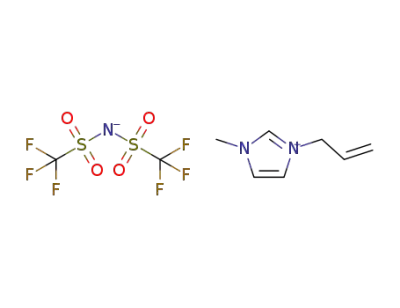
1-allyl-3-methylimidazol-3-ium N-bis(trifluoromethanesulfonyl)imidate
-
917956-73-1
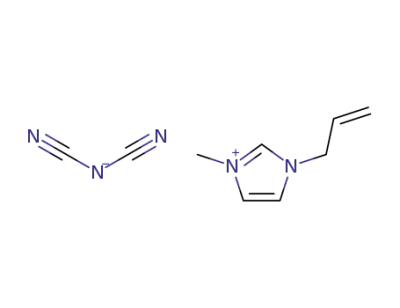
1-allyl-3-methylimidazolium dicyanamide
Relevant Products
-
N-ethyl-N-methylpyrrolidinium chloride
CAS:185438-12-4
-
1-Methylcyclopropanamine Hydrochloride
CAS:88887-87-0
-
4-tert-Butylthiophenol
CAS:2396-68-1