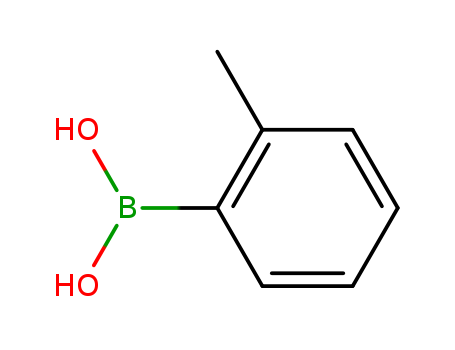
16419-60-6
- Product Name:2-Tolylboronic acid
- Molecular Formula:C7H9BO2
- Purity:99%
- Molecular Weight:135.958
Product Details;
CasNo: 16419-60-6
Molecular Formula: C7H9BO2
Appearance: beige crystalline powder
factory and supplier 16419-60-6 2-Tolylboronic acid in stock
- Molecular Formula:C7H9BO2
- Molecular Weight:135.958
- Appearance/Colour:beige crystalline powder
- Vapor Pressure:8.13E-05mmHg at 25°C
- Melting Point:162-164 °C(lit.)
- Refractive Index:1.583
- Boiling Point:283.4 °C at 760 mmHg
- PKA:8.61±0.58(Predicted)
- Flash Point:125.2 °C
- PSA:40.46000
- Density:1.1 g/cm3
- LogP:-0.32520
2-Tolylboronic acid(Cas 16419-60-6) Usage
|
Physical properties |
beige crystalline powder |
InChI:InChI=1/C7H9BO2S/c1-11-7-5-3-2-4-6(7)8(9)10/h2-5,9-10H,1H3
16419-60-6 Relevant articles
Deprotection of pinacolyl boronate esters via hydrolysis of intermediate potassium trifluoroborates
Yuen, Alexander K.L.,Hutton, Craig A.
, p. 7899 - 7903 (2005)
An efficient two-step procedure for the ...
Asymmetric construction of chiral C-N axes through rhodium-catalyzed 1,4-addition
Duan, Wei-Liang,Imazaki, Yusuke,Shintani, Ryo,Hayashi, Tamio
, p. 8529 - 8536 (2007)
Catalytic asymmetric construction of chi...
Aryllead triacetates in the synthesis of oxaphenanthrene derivatives
Fedorov, Alexey Yu,Carrara, Fabien,Finet, Jean-Pierre
, p. 5875 - 5877 (2001)
Ortho-Halomethylphenyllead triacetates (...
Structure, conformation, and dynamic processes of the stereolabile atropisomers of hindered terphenyl hydrocarbons
Lunazzi, Lodovico,Mazzanti, Andrea,Minzoni, Mirko,Edgar Anderson
, p. 1291 - 1294 (2005)
(Chemical Equation Presented) Ortho-subs...
Method for synthesizing sodium 2-carboxyphenylborate
-
Paragraph 0020-0021, (2020/06/17)
The invention discloses a method for syn...
Aryl boronic acid preparation method
-
Paragraph 0033-0036, (2020/01/25)
The invention belongs to the technical f...
Bedford-type palladacycle-catalyzed miyaura borylation of aryl halides with tetrahydroxydiboron in water
Zernickel, Anna,Du, Weiyuan,Ghorpade, Seema A.,Sawant, Dinesh N.,Makki, Arwa A.,Sekar, Nagaiyan,Eppinger, J?rg
, p. 1842 - 1851 (2018/02/23)
A mild aqueous protocol for palladium ca...
Asymmetric Arylation of Imines Catalyzed by Heterogeneous Chiral Rhodium Nanoparticles
Yasukawa, Tomohiro,Kuremoto, Tatsuya,Miyamura, Hiroyuki,Kobayashi, Sh?
supporting information, p. 2716 - 2718 (2016/06/15)
Asymmetric arylation of aldimines cataly...
16419-60-6 Process route
-
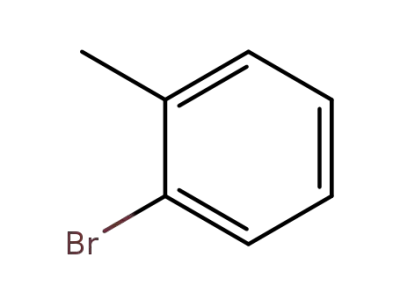
-
95-46-5
2-methylphenyl bromide

-
-
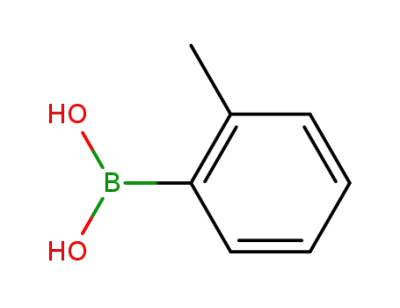
16419-60-6
2-Methylphenylboronic acid
| Conditions | Yield |
|---|---|
|
2-methylphenyl bromide;
With
n-butyllithium;
In
tetrahydrofuran;
at -78 ℃;
for 0.5h;
With
Trimethyl borate;
In
tetrahydrofuran;
at -78 - 20 ℃;
|
92% |
|
2-methylphenyl bromide;
With
tris(dibenzylideneacetone)dipalladium(0) chloroform complex; diisopropopylaminoborane; triethylamine; triphenylphosphine;
In
tetrahydrofuran;
at 65 ℃;
for 12h;
Inert atmosphere;
With
methanol;
In
tetrahydrofuran;
at 0 ℃;
Further stages;
Inert atmosphere;
|
35% |
|
With
n-butyllithium; Triisopropyl borate;
|
|
|
2-methylphenyl bromide;
With
n-butyllithium;
In
tetrahydrofuran; hexane;
at -78 ℃;
for 2h;
With
Triisopropyl borate;
In
tetrahydrofuran; hexane;
at -78 - 20 ℃;
for 2h;
With
hydrogenchloride;
In
tetrahydrofuran; hexane;
|
1.36 g |
|
With
Trimethyl borate;
|
|
|
2-methylphenyl bromide;
With
n-butyllithium;
In
tetrahydrofuran;
at -78 ℃;
for 0.5h;
With
Trimethyl borate;
In
tetrahydrofuran;
at -78 ℃;
for 0.5h;
With
hydrogenchloride;
In
tetrahydrofuran; water;
|
|
|
2-methylphenyl bromide;
With
n-butyllithium;
In
tetrahydrofuran;
at -78 ℃;
for 0.5h;
With
Trimethyl borate;
In
tetrahydrofuran;
at -78 ℃;
for 0.5h;
|
|
|
With
Trimethyl borate;
|
|
|
2-methylphenyl bromide;
With
magnesium;
In
diethyl ether;
With
Trimethyl borate;
In
diethyl ether;
In
water;
|
|
|
2-methylphenyl bromide;
With
n-butyllithium; 1,1'-bipyridyl;
In
tetrahydrofuran; hexane;
at -78 ℃;
for 4h;
With
Triisopropyl borate;
In
tetrahydrofuran; diethyl ether; hexane;
at -78 - 20 ℃;
Further stages.;
|
|
|
2-methylphenyl bromide;
With
n-butyllithium;
In
tetrahydrofuran;
at -78 ℃;
for 0.25h;
With
Trimethyl borate;
In
tetrahydrofuran;
at -78 - 20 ℃;
With
hydrogenchloride; water;
at 0 ℃;
Further stages.;
|
|
|
With
Trimethyl borate;
|
|
|
2-methylphenyl bromide;
With
magnesium;
In
diethyl ether;
at 25 ℃;
With
Trimethyl borate;
In
diethyl ether;
at -78 - 20 ℃;
for 4h;
With
water;
|
|
|
2-methylphenyl bromide;
With
n-butyllithium;
In
tetrahydrofuran;
at -78 ℃;
for 3h;
With
Trimethyl borate;
In
tetrahydrofuran;
at -78 - 20 ℃;
With
hydrogenchloride;
In
tetrahydrofuran; water;
for 1h;
|
|
|
Multi-step reaction with 2 steps
1.1: n-butyllithium / tetrahydrofuran; pentane / 1.42 h / -78 °C / Inert atmosphere
1.2: 2 h / -78 - 20 °C / Inert atmosphere
2.1: hydrogenchloride; water / Inert atmosphere
With
hydrogenchloride; n-butyllithium; water;
In
tetrahydrofuran; pentane;
|
|
|
With
tetrahydroxydiboron; (chloro(2-dicyclohexylphosphino-2′,4′,6′-triisopropyl-1,1′-biphenyl)[2-(2′-amino-1,1′-biphenyl)]palladium(II)); potassium acetate; XPhos;
In
ethanol;
at 80 ℃;
for 9h;
Inert atmosphere;
|
|
|
With
methanol; tetrakis(dimethylamido)diborane; chloro(2-dicyclohexylphosphino-2′,4′,6′-triisopropyl-1,1′-biphenyl)[2-(2′-amino-1,1′-biphenyl)]palladium(II); potassium acetate; XPhos;
at 20 - 60 ℃;
for 26.0833h;
Inert atmosphere;
Sealed tube;
|
|
|
Multi-step reaction with 2 steps
1.1: n-butyllithium / tetrahydrofuran / 1 h / -78 °C / Inert atmosphere; Schlenk technique
1.2: -78 - 20 °C / Inert atmosphere; Schlenk technique
2.1: hydrogenchloride / water / 20 °C / Inert atmosphere; Schlenk technique
With
hydrogenchloride; n-butyllithium;
In
tetrahydrofuran; water;
|
|
|
With
tetrahydroxydiboron; 1,3-bis[(diphenylphosphino)propane]dichloronickel(II); N-ethyl-N,N-diisopropylamine; triphenylphosphine;
In
ethanol;
at 80 ℃;
for 3h;
Inert atmosphere;
Sealed tube;
|
|
|
2-methylphenyl bromide;
With
magnesium;
In
tetrahydrofuran;
at 20 ℃;
for 3h;
With
Trimethyl borate;
In
tetrahydrofuran;
at -78 - 20 ℃;
In
toluene;
for 1h;
Reflux;
Dean-Stark;
|
-
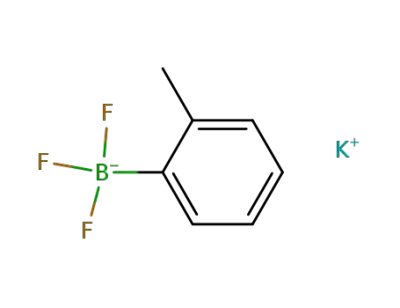
-
potassium o-tolyltrifluoroborate

-

-
16419-60-6
2-Methylphenylboronic acid
| Conditions | Yield |
|---|---|
|
With
iron(III) chloride; water;
In
tetrahydrofuran;
at 20 ℃;
for 0.5h;
|
92% |
|
With
water; silica gel;
at 20 ℃;
for 1h;
Inert atmosphere;
|
61% |
16419-60-6 Upstream products
-
932-31-0
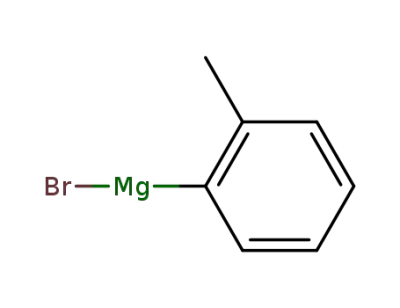
ortho-tolylmagnesium bromide
-
13195-76-1
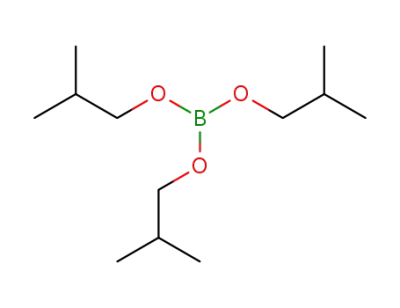
triisobutyl borate
-
4250-48-0

(2-methylphenyl)dichloroborane
-
121-43-7
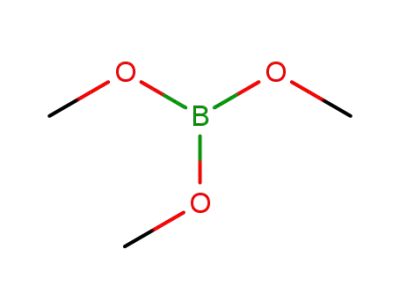
Trimethyl borate
16419-60-6 Downstream products
-
110180-68-2
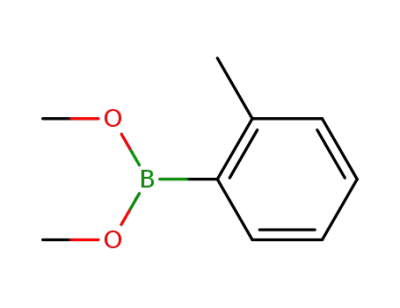
dimethoxy-o-tolyl-borane
-
7294-50-0
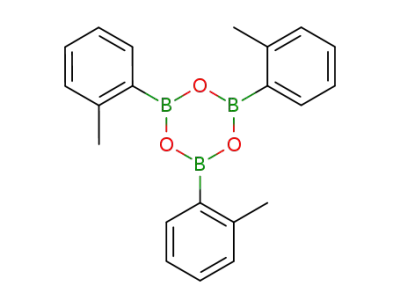
tri-o-tolylboroxine
-
91983-14-1
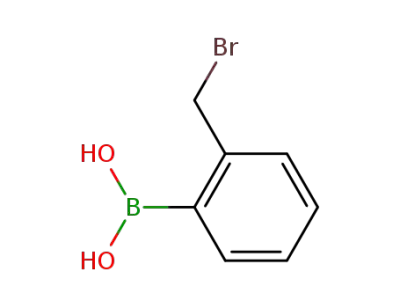
2-bromomethylphenylboronic acid
-
112627-02-8
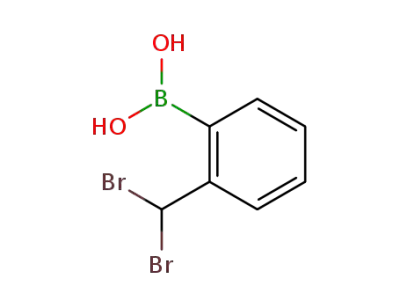
(2-Br2CHC6H4)B(OH)2
Relevant Products
-
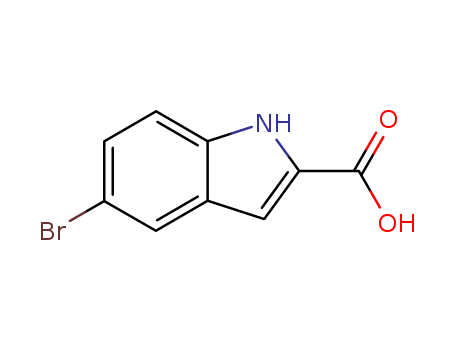
5-Bromoindole-2-carboxylic acid
CAS:7254-19-5
-
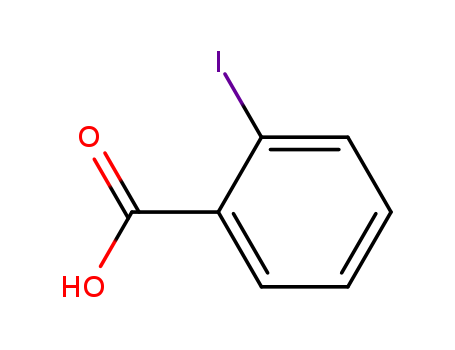
2-Iodobenzoic acid
CAS:88-67-5
-
DIBASIC ESTER
CAS:95481-62-2