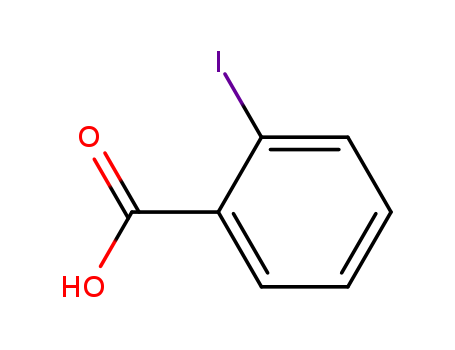
88-67-5
- Product Name:2-Iodobenzoic acid
- Molecular Formula:C7H5IO2
- Purity:99%
- Molecular Weight:248.02
Product Details;
CasNo: 88-67-5
Molecular Formula: C7H5IO2
Appearance: white crystal powder
factory and supplier 88-67-5 2-Iodobenzoic acid in stock
- Molecular Formula:C7H5IO2
- Molecular Weight:248.02
- Appearance/Colour:white crystal powder
- Vapor Pressure:0.000205mmHg at 25°C
- Melting Point:160-164 2 °C
- Boiling Point:313.9 °C at 760 mmHg
- PKA:2.85(at 25℃)
- Flash Point:143.6 °C
- PSA:37.30000
- Density:1.999 g/cm3
- LogP:1.98940
2-Iodobenzoic acid(Cas 88-67-5) Usage
|
Preparation |
2-Iodobenzoic acid is obtained from Anthranilic acid by diazotization and substitution. Diazotization of Anthranilic acid with sodium nitrite in the presence of sulfuric acid, control the temperature below 10℃, filter the diazotization solution, add the mixture of potassium iodide and sulfuric acid, stir for 10min after addition, and boil. Filter, wash with sodium thiosulfate solution, 2-Iodobenzoic acid can be recrystallized from water. |
|
Synthesis Reference(s) |
Journal of the American Chemical Society, 93, p. 4841, 1971 DOI: 10.1021/ja00748a029 |
|
Purification Methods |
Crystallise the acid repeatedly from water and EtOH. Sublime it under vacuum at 100o. [Beilstein 9 IV 1030.] |
|
General Description |
**2-Iodobenzoic acid** is a chemical compound used as a reagent in organic synthesis, particularly in Cu-catalyzed coupling-cyclization reactions to form isocoumarins under greener conditions. It serves as a key substrate in ultrasound-mediated reactions, where it reacts with terminal alkynes in the presence of a CuI–K2CO3-PEG 400 system, demonstrating high regioselectivity and sustainability by avoiding toxic solvents and palladium catalysts. Its applications extend to pharmaceutical research, contributing to the synthesis of biologically active heterocycles. *(No relevant pharmacological conclusions from the first abstract; focus is on synthetic utility.)* |
|
Definition |
ChEBI: An iodobenzoic acid with a single iodo substituent placed at the 2-position. |
InChI:InChI=1/C7H5IO2/c8-6-4-2-1-3-5(6)7(9)10/h1-4H,(H,9,10)/p-1
88-67-5 Relevant articles
Perfluoroalkyl Cobaloximes: Preparation Using Hypervalent Iodine Reagents, Molecular Structures, Thermal and Photochemical Reactivity
Liebing, Phil,Oehler, Florian,Wagner, Mona,Tripet, Pascal F.,Togni, Antonio
, p. 570 - 583 (2018)
Treatment of cobaloximes(II), [Co(Hdmg)2...
N-Alkenylation of hydroxamic acid derivatives with ethynyl benziodoxolone to synthesizecis-enamides through vinyl benziodoxolones
Shimbo, Daisuke,Maruyama, Toshifumi,Tada, Norihiro,Itoh, Akichika
supporting information, p. 2442 - 2447 (2021/04/02)
The stereoselective synthesis ofcis-β-N-...
Aerobic oxidation of aldehydes to carboxylic acids catalyzed by recyclable ag/c3 n4 catalyst
Wu, Chaolong,Yao, Xiaoquan,Yu, Min,Zhou, Li,Zhu, Li
, p. 167 - 175 (2021/03/19)
The oxidation of aldehydes is an efficie...
Mechanistic Investigation of the Iron-Catalyzed Azidation of Alkyl C(sp3)-H Bonds with Zhdankin’s λ3-Azidoiodane
Chatterjee, Ruchira,Day, Craig S.,Fawcett, Alexander,Hartwig, John F.
supporting information, p. 16184 - 16196 (2021/10/12)
An in-depth study of the mechanism of th...
Togni-II Reagent Mediated Selective Hydrotrifluoromethylation and Hydrothiolation of Alkenes?
Teng, Shuang,Meng, Lingkui,Xu, Bingbing,Tu, Guangsheng,Wu, Peng,Liao, Zhiwen,Tan, Yulin,Guo, Jian,Zeng, Jing,Wan, Qian
supporting information, p. 3429 - 3434 (2021/11/08)
Based on the redox reactions of Togni-II...
88-67-5 Process route
-
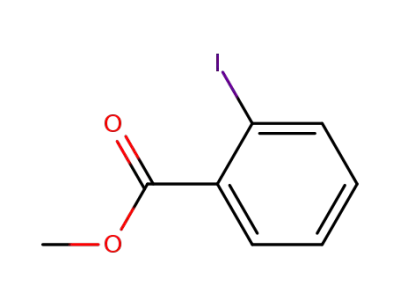
-
610-97-9
o-iodo-methyl-benzoic acid

-
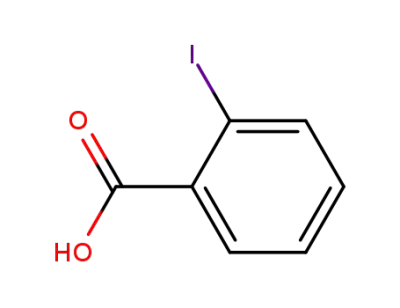
-
88-67-5
2-Iodobenzoic acid

-
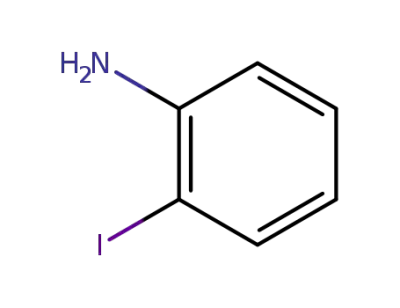
-
615-43-0
2-iodophenylamine
| Conditions | Yield |
|---|---|
|
With
hydroxylamine hydrochloride; 1,8-diazabicyclo[5.4.0]undec-7-ene;
In
dimethyl sulfoxide;
at 25 - 90 ℃;
for 7h;
|
7% 45% |
-
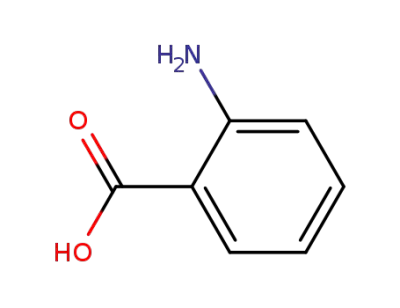
-
118-92-3,159201-01-1,50816-84-7,80206-34-4,104809-47-4
anthranilic acid

-

-
88-67-5
2-Iodobenzoic acid
| Conditions | Yield |
|---|---|
|
anthranilic acid;
With
sodium hydrogen sulfate; sodium nitrite;
In
water;
at 20 ℃;
for 0.166667h;
Paste grinding;
With
potassium iodide;
In
water;
at 20 ℃;
for 0.333333h;
Paste grinding;
|
80% |
|
With
N-iodo-succinimide; sodium nitrite;
In
N,N-dimethyl-formamide;
at 20 ℃;
for 4h;
|
78% |
|
anthranilic acid;
With
sulfuric acid; silica gel; sodium nitrite;
at 20 ℃;
grinding;
Neat (no solvent);
With
water;
at 20 ℃;
grinding;
Neat (no solvent);
With
potassium iodide;
at 20 ℃;
for 0.166667h;
grinding;
Neat (no solvent);
|
74% |
|
anthranilic acid;
With
toluene-4-sulfonic acid;
In
water;
at 20 ℃;
With
potassium iodide;
In
water;
at 20 ℃;
for 5h;
|
74% |
|
anthranilic acid;
With
cation-exchange resin KU-2-8; sodium nitrite;
In
water;
at 20 ℃;
for 3h;
With
potassium iodide;
In
water;
at 20 ℃;
for 3.16667h;
Further stages.;
|
65% |
|
With
hydrogenchloride; potassium iodide; sodium nitrite;
In
dichloromethane;
at 0 - 20 ℃;
for 4h;
|
55% |
|
anthranilic acid;
With
water; sodium nitrite;
In
neat (no solvent);
at 20 ℃;
With
potassium iodide;
In
neat (no solvent);
at 20 ℃;
|
55% |
|
With
toluene-4-sulfonic acid; potassium iodide; sodium nitrite;
In
acetonitrile;
at 20 ℃;
for 0.5h;
|
50% |
|
anthranilic acid;
With
nicotinic acid sulfate; sodium nitrite;
In
water;
at 20 ℃;
for 0.5h;
Grinding;
With
sodium iodide;
In
water;
at 20 ℃;
|
47% |
|
durch Austausch von NH2 gegen Jod;
|
|
|
With
sulfuric acid;
man diazotiert mit NaNO2 in Wasser, giesst in eine Mischung von KI, verd. Schwefelsaeure und Wasser;
|
|
|
|
|
|
anthranilic acid;
With
potassium iodide;
|
|
|
anthranilic acid;
With
sulfuric acid; copper; copper(II) sulfate; potassium iodide;
In
water;
for 2h;
Reflux;
With
sodium nitrite;
In
water;
at 80 - 90 ℃;
|
|
|
anthranilic acid;
With
hydrogenchloride; sodium nitrite;
In
water;
at 0 ℃;
for 1h;
With
potassium iodide;
In
water;
at 0 - 90 ℃;
|
|
|
anthranilic acid;
With
hydrogenchloride; sodium nitrite;
In
water;
at 0 - 5 ℃;
for 0.5h;
Inert atmosphere;
With
sulfuric acid; potassium iodide;
In
water;
at 10 - 90 ℃;
for 1h;
Inert atmosphere;
|
|
|
anthranilic acid;
With
sulfuric acid; sodium nitrite;
In
water;
at 5 ℃;
for 0.5h;
Inert atmosphere;
With
sulfuric acid; potassium iodide;
In
water;
at 100 ℃;
for 1h;
Inert atmosphere;
|
|
|
anthranilic acid;
With
hydrogenchloride; sodium nitrite;
In
water;
at 0 ℃;
for 0.5h;
With
potassium iodide;
In
water;
at 0 - 90 ℃;
for 1.83333h;
|
|
|
anthranilic acid;
With
hydrogenchloride; sodium nitrite;
In
water;
at 0 ℃;
With
potassium iodide;
In
water;
at 0 - 90 ℃;
for 1h;
|
88-67-5 Upstream products
-
645-00-1
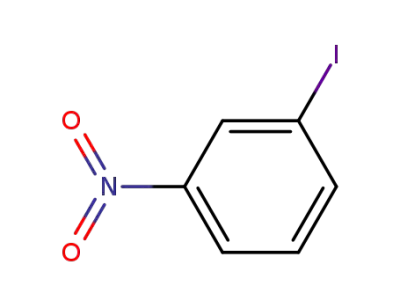
m-iodonitrobenzene
-
151-50-8
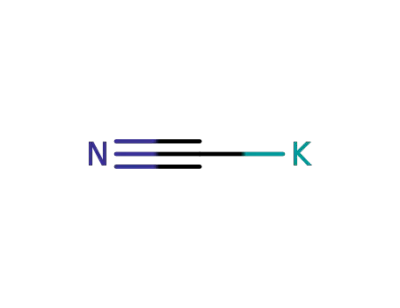
potassium cyanide
-
615-37-2
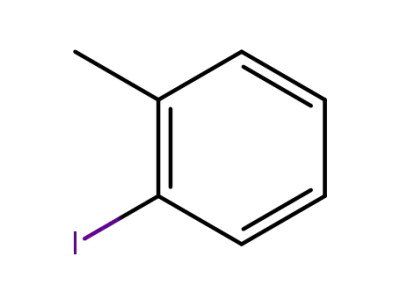
ortho-methylphenyl iodide
-
615-42-9
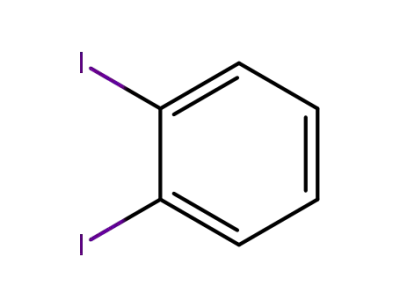
1,2-Diiodobenzene
88-67-5 Downstream products
-
610-97-9

o-iodo-methyl-benzoic acid
-
1829-26-1
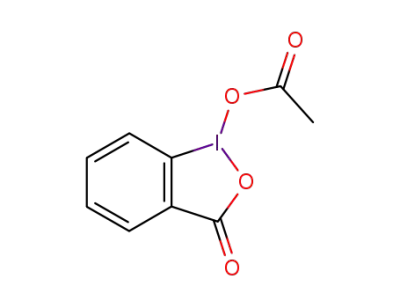
1-acetoxy-1,2-benziodoxol-3-one
-
40535-46-4
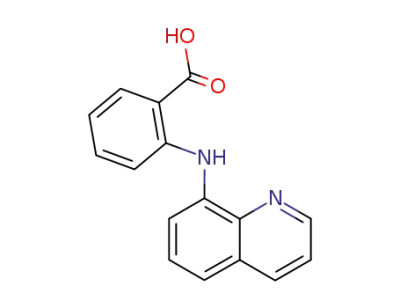
Ν-(quinolinyl)-o-aminobenzoic acid
-
88-99-3
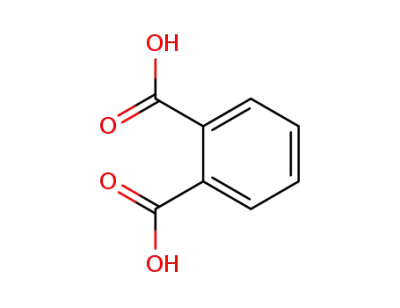
benzene-1,2-dicarboxylic acid
Relevant Products
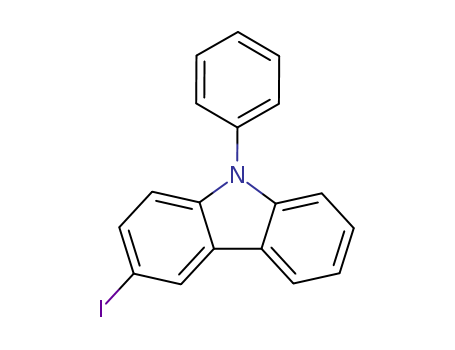
-
3-Iodo-N-phenylcarbazole
CAS:502161-03-7
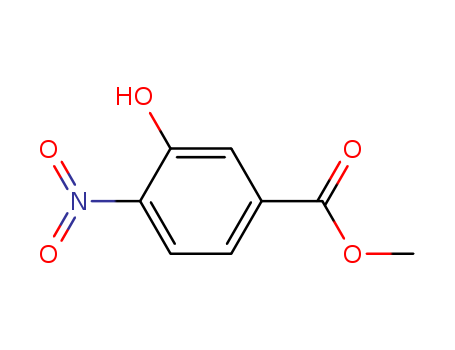
-
Methyl 3-hydroxy-4-nitrobenzoate
CAS:713-52-0
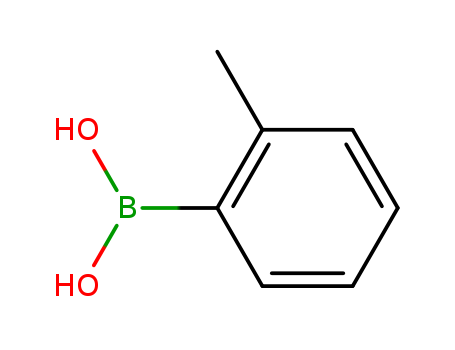
-
2-Tolylboronic acid
CAS:16419-60-6