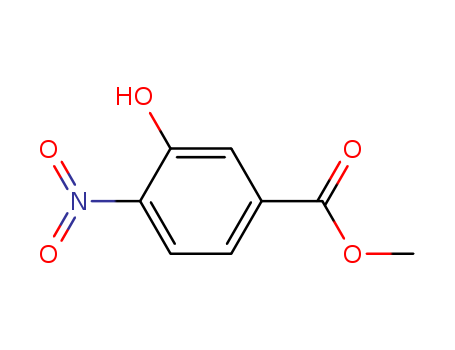
713-52-0
- Product Name:Methyl 3-hydroxy-4-nitrobenzoate
- Molecular Formula:C8H7NO5
- Purity:99%
- Molecular Weight:197.147
Product Details;
CasNo: 713-52-0
Molecular Formula: C8H7NO5
factory and supplier 713-52-0 Methyl 3-hydroxy-4-nitrobenzoate in stock
- Molecular Formula:C8H7NO5
- Molecular Weight:197.147
- Vapor Pressure:2.88E-05mmHg at 25°C
- Melting Point:90-91 °C
- Boiling Point:346.4 °C at 760 mmHg
- PKA:6.05±0.13(Predicted)
- Flash Point:163.3 °C
- PSA:92.35000
- Density:1.432 g/cm3
- LogP:1.61020
Methyl 3-hydroxy-4-nitrobenzoate(Cas 713-52-0) Usage
|
General Description |
Methyl 3-hydroxy-4-nitrobenzoate, also known as methyl 3-hydroxy-4-nitrobenzoate, is a chemical compound with the molecular formula C8H7NO5. It is a yellow crystalline solid that is commonly used in the production of pharmaceuticals and organic synthesis. Methyl 3-hydroxy-4-nitrobenzoate is an ester of methyl alcohol and 3-hydroxy-4-nitrobenzoic acid, and it is often used as a building block for the synthesis of various other organic compounds. Methyl 3-hydroxy-4-nitrobenzoate has potential applications in the pharmaceutical industry due to its pharmacological properties, and it is also used as an intermediate in the manufacturing of dyes and pigments. Additionally, it is important to handle and store this compound carefully, as it can be hazardous if not properly managed. |
InChI:InChI=1/C8H7NO5/c1-14-8(11)5-2-3-6(9(12)13)7(10)4-5/h2-4,10H,1H3/p-1
713-52-0 Relevant articles
Pinpointing mechanochemical bond rupture by embedding the mechanophore into a macrocycle
Schütze, Doreen,Holz, Katharina,Müller, Julian,Beyer, Martin K.,Lüning, Ulrich,Hartke, Bernd
, p. 2556 - 2559 (2015)
Mechanophores contain a mechanically lab...
Photoconductive bent-core liquid crystalline radicals with a paramagnetic polar switchable phase
Shivakumar, Kilingaru I.,Pociecha, Damian,Szczytko, Jacek,Kapu?ciński, Szymon,Monobe, Hirosato,Kaszyński, Piotr
supporting information, p. 1083 - 1088 (2020/02/05)
A series of self-organizing bent-core de...
Discovery of new ATP-competitive inhibitors of human DNA topoisomerase IIα through screening of bacterial topoisomerase inhibitors
Baran?oková, Michaela,Durcik, Martina,Gramec Skledar, Darja,Ila?, Janez,Kikelj, Danijel,Peterlin Ma?i?, Lucija,Skok, ?iga,Toma?i?, Tihomir,Zega, Anamarija,Zidar, Nace
, (2020/07/21)
Human DNA topoisomerase II is one of the...
NEW CLASS OF DNA GYRASE AND/OR TOPOISOMERASE IV INHIBITORS WITH ACTIVITY AGAINST GRAM-POSITIVE AND GRAM-NEGATIVE BACTERIA
-
Page/Page column 58, (2020/03/29)
The present invention relates to compoun...
Aromatic acyl hydrazone derivative and application thereof as NA inhibitor
-
Paragraph 0039-0043; 0133-0137, (2020/12/30)
The invention relates to an aromatic acy...
713-52-0 Process route
-
-
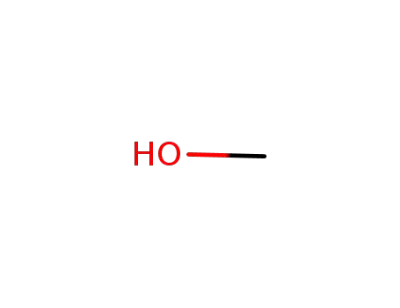
67-56-1
methanol

-
-
619-14-7
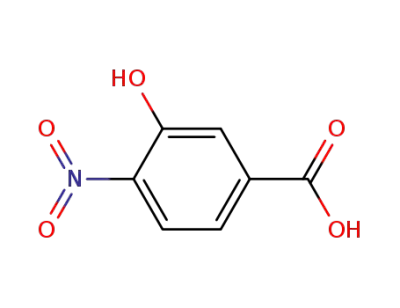
3-hydroxy-4-nitro-benzoic acid

-
-
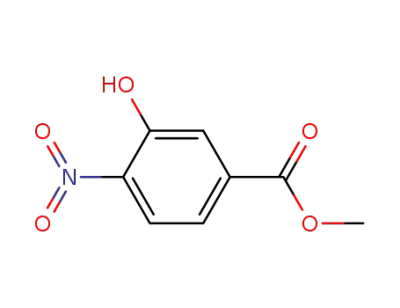
713-52-0
methyl 3-hydroxy-4-nitrobenzoate
| Conditions | Yield |
|---|---|
|
With
sulfuric acid;
at 65 ℃;
for 17h;
|
100% |
|
With
thionyl chloride;
at 0 ℃;
for 12h;
Reflux;
Inert atmosphere;
|
100% |
|
With
thionyl chloride;
at 0 ℃;
for 17h;
Reflux;
Inert atmosphere;
|
100% |
|
With
thionyl chloride;
at 0 - 70 ℃;
for 1h;
|
100% |
|
With
sulfuric acid;
for 5h;
Heating;
|
99% |
|
With
sulfuric acid;
at 65 ℃;
Inert atmosphere;
|
99% |
|
With
sulfuric acid;
In
water;
at 60 ℃;
for 144h;
|
99% |
|
With
sulfuric acid;
for 18h;
Reflux;
|
99% |
|
With
sulfuric acid;
for 24h;
Reflux;
Inert atmosphere;
|
99% |
|
With
sulfuric acid;
Reflux;
|
99% |
|
With
sulfuric acid;
for 5h;
Reflux;
|
99.6% |
|
With
sulfuric acid;
Reflux;
|
97% |
|
With
thionyl chloride;
at 0 - 20 ℃;
for 15h;
|
97% |
|
With
thionyl chloride;
at 0 ℃;
for 2h;
Reflux;
Inert atmosphere;
|
96.7% |
|
With
thionyl chloride;
at 0 ℃;
for 2h;
Reflux;
|
96.7% |
|
With
thionyl chloride;
for 0.5h;
Heating / reflux;
|
95% |
|
With
thionyl chloride;
Inert atmosphere;
|
95% |
|
With
sulfuric acid;
at 60 ℃;
|
94% |
|
With
sulfuric acid;
for 16h;
Reflux;
|
94% |
|
With
sulfuric acid;
for 15h;
Reflux;
|
93% |
|
With
thionyl chloride;
at 0 - 20 ℃;
for 15h;
|
92% |
|
With
toluene-4-sulfonic acid;
at 65 ℃;
for 60h;
Inert atmosphere;
|
90.9% |
|
With
thionyl chloride;
at 70 ℃;
for 4h;
Inert atmosphere;
|
90% |
|
With
sulfuric acid;
Heating;
|
70% |
|
With
sulfuric acid;
|
|
|
With
sulfuric acid;
for 48h;
Heating;
|
|
|
With
toluene-4-sulfonic acid;
at 65 ℃;
for 60h;
|
2.38 g |
|
With
sulfuric acid;
Heating;
|
|
|
With
sulfuric acid;
at 60 ℃;
for 18h;
|
|
|
With
sulfuric acid;
at 65 ℃;
|
|
|
With
sulfuric acid;
at 65 ℃;
|
|
|
With
thionyl chloride;
at 0 ℃;
for 1.5h;
Inert atmosphere;
Reflux;
|
|
|
With
toluene-4-sulfonic acid;
for 12h;
Reflux;
|
|
|
With
thionyl chloride;
at 20 ℃;
Schlenk technique;
|
|
|
With
sulfuric acid;
|
|
|
With
thionyl chloride;
at 0 - 20 ℃;
for 15h;
|
|
|
With
sulfuric acid;
for 18h;
Reflux;
|
99 mg |
|
With
thionyl chloride;
at 65 ℃;
|
|
|
With
thionyl chloride;
at 20 ℃;
Schlenk technique;
|
-

-
619-14-7
3-hydroxy-4-nitro-benzoic acid

-
-
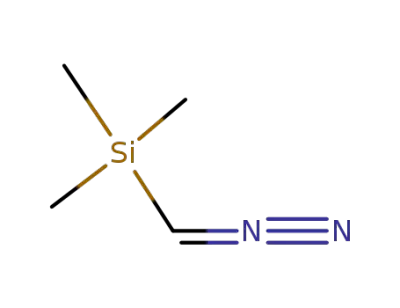
18107-18-1
diazomethyl-trimethyl-silane

-

-
713-52-0
methyl 3-hydroxy-4-nitrobenzoate
| Conditions | Yield |
|---|---|
|
In
methanol; diethyl ether; toluene;
at 0 ℃;
for 0.5h;
Inert atmosphere;
|
90% |
713-52-0 Upstream products
-
67-56-1

methanol
-
619-14-7

3-hydroxy-4-nitro-benzoic acid
-
326799-92-2
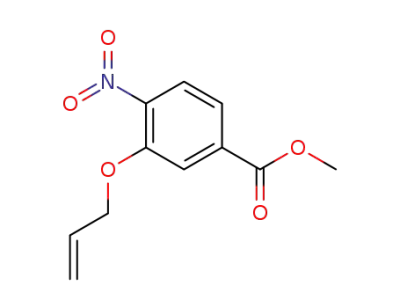
methyl 4-nitro-3-(prop-2-en-1-yloxy)benzoate
-
75-36-5

acetyl chloride
713-52-0 Downstream products
-
185540-61-8
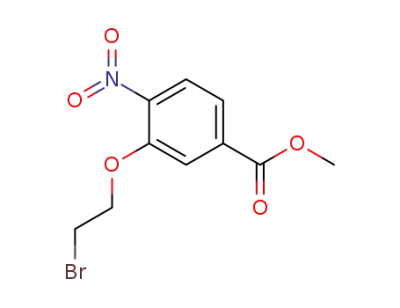
methyl 3-(2-bromoethoxy)-4-nitrobenzoate
-
61161-83-9
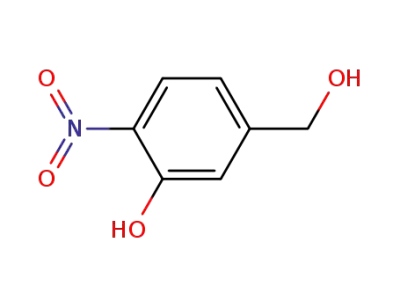
5-(hydroxymethyl)-2-nitrophenol
-
183430-73-1
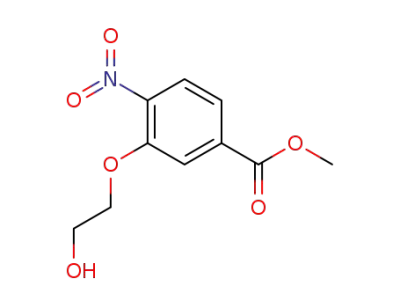
methyl 3-(2-hydroxyethoxy)-4-nitrobenzoate
-
330206-41-2
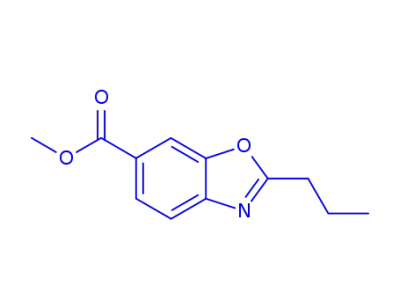
6-carbomethoxy-2-propylbenzoxazole
Relevant Products
-
2,5-Dimethoxy-Beta-Nitrostyrene
CAS:40276-11-7
-
3-bromo-9-phenyl-9H-carbazole
CAS:1153-85-1
-
2-Iodobenzoic acid
CAS:88-67-5