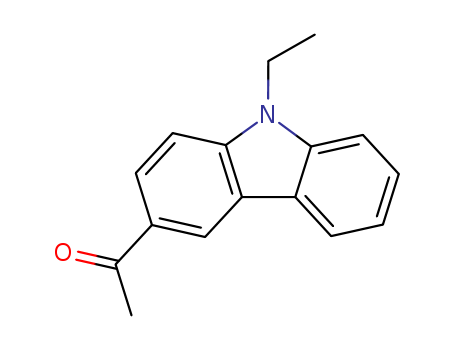
1484-04-4
- Product Name:1-(9-Ethyl-9H-carbazol-3-yl)ethan-1-one
- Molecular Formula:C16H15NO
- Purity:99%
- Molecular Weight:237.301
Product Details;
CasNo: 1484-04-4
Molecular Formula: C16H15NO
factory and supplier 1484-04-4 1-(9-Ethyl-9H-carbazol-3-yl)ethan-1-one in stock
- Molecular Formula:C16H15NO
- Molecular Weight:237.301
- Vapor Pressure:3.58E-05mmHg at 25°C
- Melting Point:115 °C
- Boiling Point:353.5oC at 760 mmHg
- Flash Point:167.6oC
- PSA:22.00000
- Density:1.12g/cm3
- LogP:4.01700
1-(9-ETHYL-9H-CARBAZOL-3-YL)ETHANONE(Cas 1484-04-4) Usage
|
General Description |
1-(9-Ethyl-9H-carbazol-3-yl)ethanone, also known as Ethylcarbazole ketone, is a chemical compound with the molecular formula C17H15NO. It is a ketone derivative of carbazole, which is a heterocyclic aromatic organic compound. Ethylcarbazole ketone is widely used as a reactant in organic synthesis and as an intermediate in the production of various pharmaceuticals, agrochemicals, and dyes. It has also been studied for its potential applications in the development of organic electronic materials and optoelectronic devices. Additionally, it possesses antioxidant and anti-inflammatory properties, making it a potential candidate for the development of new drugs. 1-(9-ETHYL-9H-CARBAZOL-3-YL)ETHANONE should be handled and used with care, following proper safety precautions and guidelines for handling hazardous chemicals. |
InChI:InChI=1/C16H15NO/c1-3-17-15-7-5-4-6-13(15)14-10-12(11(2)18)8-9-16(14)17/h4-10H,3H2,1-2H3
1484-04-4 Relevant articles
A short route to heteroarylcarbazoles: Synthesis of new pyrazolylcarbazoles and carbazolylquinolines
Nagarajan, Rajagopal,Perumal, Paramasivan T.
, p. 1269 - 1273 (2004)
A short synthesis of pyrazolylcarbazoles...
Facile procedure for the synthesis of 3-acetyl-9-ethylcarbazole and corresponding functionalized bis-diketone compounds
Tang, Rui-Ren,Zhang, Wei
, p. 601 - 606 (2010)
Acetylation-substituted N-ethylcarbazole...
A New and Facile Method for the Synthesis of Nitrocarbazoles by Urea Nitrate
Nagarajan, Rajagopal,Muralidharan,Perumal, Paramasivan T.
, p. 1259 - 1264 (2004)
Urea nitrate in acetic acid is found to ...
Pure Organic Room Temperature Phosphorescence from Excited Dimers in Self-Assembled Nanoparticles under Visible and Near-Infrared Irradiation in Water
Wang, Xiao-Fang,Xiao, Hongyan,Chen, Peng-Zhong,Yang, Qing-Zheng,Chen, Bin,Tung, Chen-Ho,Chen, Yu-Zhe,Wu, Li-Zhu
, p. 5045 - 5050 (2019)
Pure organic room temperature phosphores...
A facile method for the synthesis of acetylcarbazoles and carbazole aldehydes
Nagarajan, Rajagopal,Perumal, Paramasivan T.
, p. 2127 - 2133 (2004)
Acetylation of 9-alkylcarbazoles with ac...
HS? facilitated sulfur pyran realizing hydrogen sulfide detection and imaging in HepG2 cells and chlorella
Chao, Jianbin,Xu, Miao,Zhang, Yongbin,Huo, Fangjun,Liu, Yaoming,Wang, Xiaolu,Yin, Caixia
, p. 227 - 232 (2019)
The new carbazole-based fluorescent prob...
Influence of thiophene spacer and auxiliary acceptor on the optical properties of 4-(Diethylamino)-2-hydroxybenzaldehyde based D-π-A-π-D Colorants with N-alkyl donors: Experimental, DFT and Z-scan study
Raikwar, Manish M.,Patil, Dinesh S.,Mathew, Elizabeth,Varghese, Manu,Joe, Issac H.,Sekar, Nagaiyan
, p. 45 - 58 (2019)
We report here design and synthesis of f...
A Solid-State Fluorescent Material Based on Carbazole-Containing Difluoroboron β-Diketonate: Multiple Chromisms, the Self-Assembly Behavior, and Optical Waveguides
Chen, Peng-Zhong,Zhang, Han,Niu, Li-Ya,Zhang, Yi,Chen, Yu-Zhe,Fu, Hong-Bing,Yang, Qing-Zheng
, (2017)
A carbazole-containing difluoroboron β-d...
Fluorescent probe of reversible sulfur dioxide/sulphurous acid (hydrogen) salt
-
Paragraph 0025; 0026, (2018/06/16)
The invention provides a fluorescent pro...
1484-04-4 Process route
-
-
86-28-2
N-ethylcarbazole

-
-
108-24-7
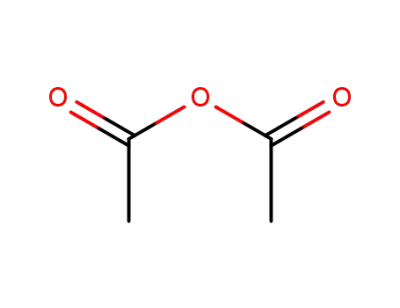
acetic anhydride

-
-
1484-04-4
N-ethyl-3-acetylcarbazole
| Conditions | Yield |
|---|---|
|
N-ethylcarbazole; acetic anhydride;
With
boron trifluoride diethyl etherate;
at 20 ℃;
for 4h;
With
hydrogenchloride; water;
|
80.4% |
|
With
aluminum (III) chloride;
In
dichloromethane;
at 0 - 20 ℃;
|
70% |
|
With
aluminum (III) chloride;
In
dichloromethane;
at 20 ℃;
for 4h;
|
70.47% |
|
With
perchloric acid; triphenylphosphine;
In
dichloromethane;
at 20 ℃;
for 2h;
|
61% |
|
With
bismuth(III) chloride;
In
dichloromethane;
at 20 ℃;
for 0.583333h;
|
60% |
|
With
In(OSO2CF3)3;
In
dichloromethane;
for 2.5h;
|
59% |
|
N-ethylcarbazole;
With
aluminum (III) chloride;
In
dichloromethane;
at 20 ℃;
for 0.5h;
acetic anhydride;
In
dichloromethane;
at 0 - 20 ℃;
for 6h;
|
-

-
86-28-2
N-ethylcarbazole

-
-
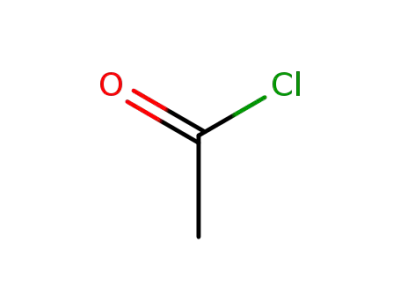
75-36-5
acetyl chloride

-

-
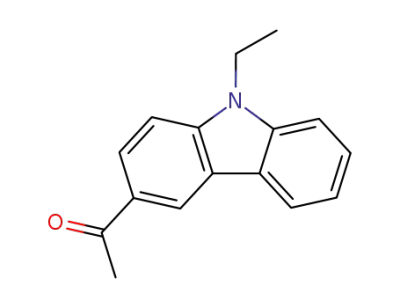
1484-04-4
N-ethyl-3-acetylcarbazole
| Conditions | Yield |
|---|---|
|
With
bismuth(III) chloride;
In
dichloromethane;
at 20 ℃;
|
81% |
|
With
zinc(II) chloride;
In
dichloromethane;
at 20 ℃;
for 6h;
|
80% |
|
With
zinc(II) chloride;
|
|
|
With
zinc(II) chloride;
In
dichloromethane;
at 20 ℃;
for 24h;
|
|
|
With
aluminum (III) chloride;
In
dichloromethane;
at 20 ℃;
for 4h;
Cooling with ice;
|
|
|
With
zinc(II) chloride;
In
dichloromethane;
for 5h;
Reflux;
|
|
|
With
aluminum (III) chloride;
In
dichloromethane;
|
1484-04-4 Upstream products
-
86-28-2

N-ethylcarbazole
-
75-36-5

acetyl chloride
-
108-24-7

acetic anhydride
-
86-74-8
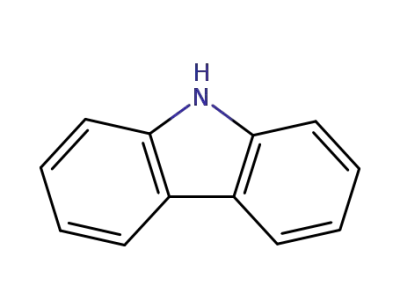
9H-carbazole
1484-04-4 Downstream products
-
879212-03-0
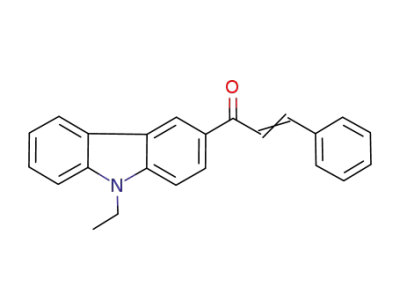
1‐(9‐ethyl‐9H‐carbazol‐3‐yl)‐3‐phenylprop‐2‐en‐1‐one
-
1191034-17-9
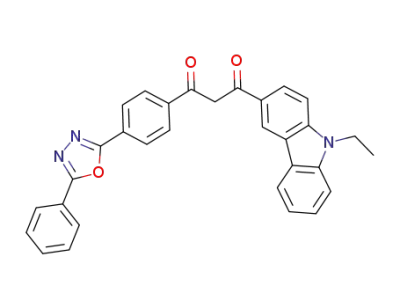
1-(9-ethyl-9H-carbazol-3-yl)-3-[4-(5-phenyl-1,3,4-oxadiazol-2-yl)phenyl]propane-1,3-dione
-
1219036-84-6
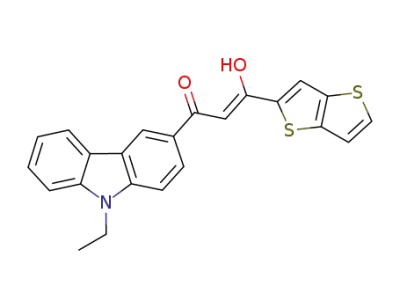
(2Z)-1-(9-ethyl-9H-carbazol-2-yl)-3-hydroxy-3-thieno[3,2-b]thiophen-2-ylprop-2-en-1-one
-
1239875-14-9
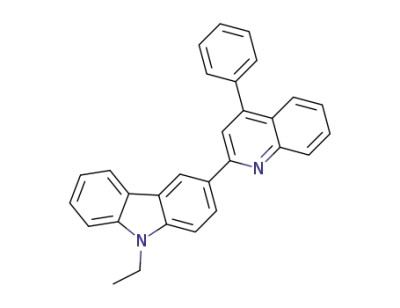
9-ethyl-3-(4-phenylquinolin-2-yl)-9H-carbazole
Relevant Products
-
2,5-Dimethoxy-Beta-Nitrostyrene
CAS:40276-11-7
-
N,N'-Bis(salicylidene)-1,3-propanediamine
CAS:120-70-7
-
N-ethylenecarbazole
CAS:1484-13-5