13716-12-6
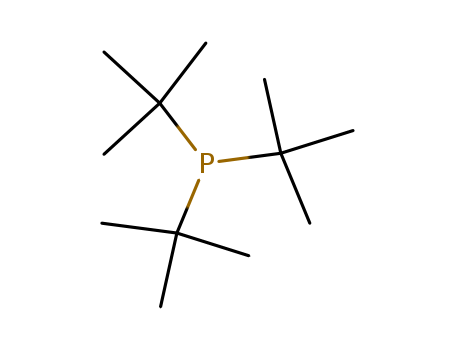
- Product Name:Tri-tert-butylphosphine
- Molecular Formula:C12H27P
- Purity:99%
- Molecular Weight:202.32
Product Details;
CasNo: 13716-12-6
Molecular Formula: C12H27P
Appearance: Colorless to light yellow liquid
factory and supplier 13716-12-6 Tri-tert-butylphosphine in stock
- Molecular Formula:C12H27P
- Molecular Weight:202.32
- Appearance/Colour:Colorless to light yellow liquid
- Vapor Pressure:0.105mmHg at 25°C
- Melting Point:30-35 °C(lit.)
- Boiling Point:229.4 °C at 760 mmHg
- Flash Point:94.6 °C
- PSA:13.59000
- Density:0.861 g/mL at 25 °C
- LogP:4.86380
Tri-tert-butylphosphine(Cas 13716-12-6) Usage
|
Reaction |
Useful as a ligand in a variety of palladium-catalyzed C-N, C-O and C-C bond-forming reactions under mild conditions. |
InChI:InChI=1/C12H27P/c1-10(2,3)13(11(4,5)6)12(7,8)9/h1-9H3
13716-12-6 Relevant articles
Reactivity of [Pt(PtBu3)2] with Zinc(I/II) Compounds: Bimetallic Adducts, Zn-Zn Bond Cleavage, and Cooperative Reactivity
Hidalgo, Nereida,Romero-Pérez, Carlos,Maya, Celia,Fernández, Israel,Campos, Jesús
, p. 1113 - 1119 (2021)
Metal-only Lewis pairs (MOLPs) based on ...
Raney-Ni reduction of phosphine sulfides
Demchuk, Oleg M.,?wierczyńska, Wioletta,Dziuba, Kamil,Frynas, S?awomir,Flis, Anna,Pietrusiewicz, K. Micha?
, p. 64 - 68 (2017)
A variety of tertiary phosphine sulfides...
Oxidative ring expansion of a low-coordinate palladacycle: Synthesis of a robust T-shaped alkylpalladium(II) complex
Sinclair, Matthew J.G.,Chaplin, Adrian B.
, (2020)
The synthesis of an unusual T-shaped alk...
Photochemical transformation of chlorobenzenes and white phosphorus into arylphosphines and phosphonium salts
Gschwind, Ruth M.,Mende, Michael,Scott, Daniel J.,Streitferdt, Verena,Till, Marion,Wolf, Robert
supporting information, p. 1100 - 1103 (2022/02/03)
Chlorobenzenes are important starting ma...
Process for synthesizing tri-tert-butylphosphonium tetrafluoroborate
-
Paragraph 0017-0018; 0020-0021; 0023-0024; 0026-0027; ..., (2021/11/26)
A tert-butyl Grignard reagent is reacted...
A Lewis Base Nucleofugality Parameter, NFB, and Its Application in an Analysis of MIDA-Boronate Hydrolysis Kinetics
García-Domínguez, Andrés,Gonzalez, Jorge A.,Leach, Andrew G.,Lloyd-Jones, Guy C.,Nichol, Gary S.,Taylor, Nicholas P.
supporting information, (2022/01/04)
The kinetics of quinuclidine displacemen...
13716-12-6 Process route
-
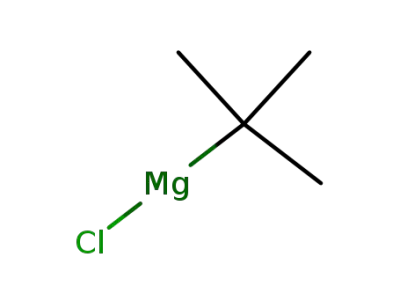
-
677-22-5
tert-butylmagnesium chloride

-
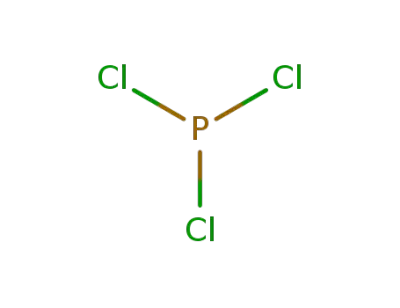
-
7719-12-2,52843-90-0
phosphorus trichloride

-
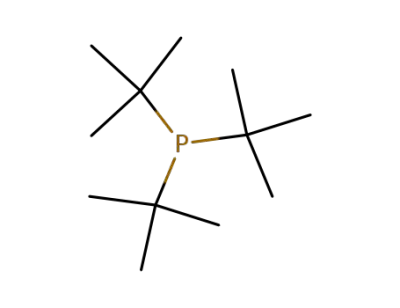
-
13716-12-6
tri-tert-butyl phosphine
| Conditions | Yield |
|---|---|
|
With
lithium bromide;
copper(l) iodide;
In
diethyl ether; hexane;
at -20 - 20 ℃;
for 4h;
|
88.3% |
-

-
677-22-5
tert-butylmagnesium chloride

-

-
13716-12-6
tri-tert-butyl phosphine
| Conditions | Yield |
|---|---|
|
With
boron trifluoride-tetrahydrofuran complex; phosphorus trichloride;
In
tetrahydrofuran;
at -10 - 0 ℃;
for 3h;
Reagent/catalyst;
Inert atmosphere;
|
94.3% |
|
With
phosphorus trichloride;
|
13716-12-6 Upstream products
-
677-22-5

tert-butylmagnesium chloride
-
13716-10-4
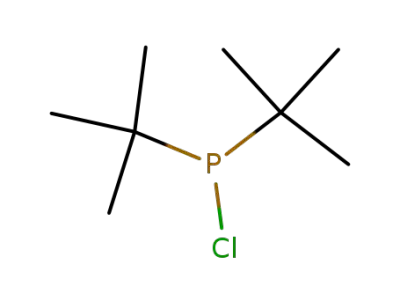
di(tert-butyl)chlorophosphine
-
594-19-4
tert.-butyl lithium
-
60483-74-1
Tri-tert-butylphosphantellurid
13716-12-6 Downstream products
-
95-46-5
2-methylphenyl bromide
-
95-49-8
2-methylchlorobenzene
-
615-37-2
ortho-methylphenyl iodide
-
108-86-1
bromobenzene
Relevant Products
-
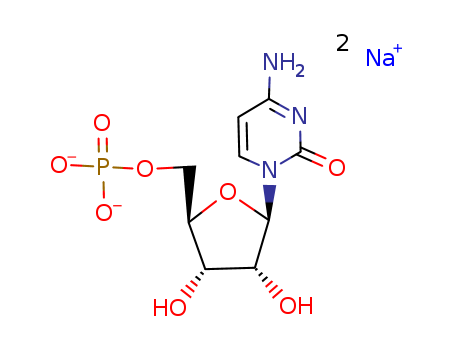
Cytidine 5'-monophosphate disodium salt
CAS:6757-06-8
-
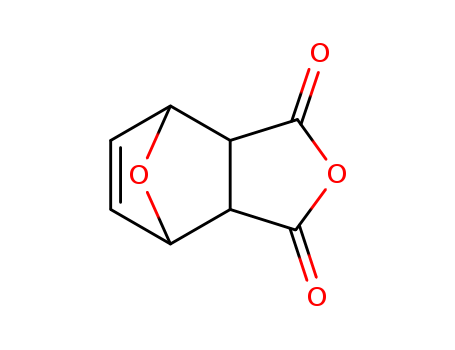
4,10-dioxatricyclo[5.2.1.0^{2,6}]dec-8-ene-3,5-dione
CAS:5426-09-5
-
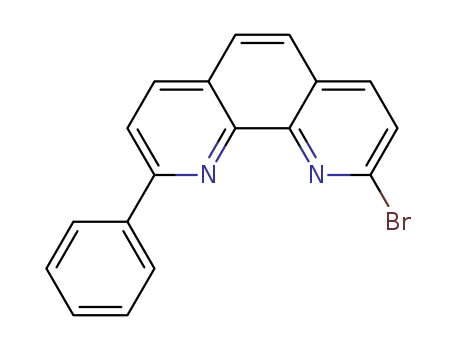
2-bromo-9-phenyl-1,10-phenanthroline
CAS:2042493-16-1