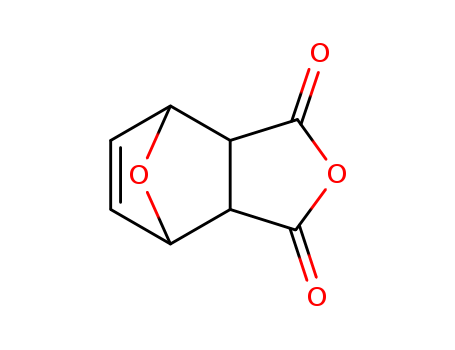
5426-09-5
- Product Name:4,10-dioxatricyclo[5.2.1.0^{2,6}]dec-8-ene-3,5-dione
- Molecular Formula:C8H6 O4
- Purity:99%
- Molecular Weight:166.133
Product Details;
CasNo: 5426-09-5
Molecular Formula: C8H6 O4
factory and supplier 5426-09-5 4,10-dioxatricyclo[5.2.1.0^{2,6}]dec-8-ene-3,5-dione in stock
- Molecular Formula:C8H6 O4
- Molecular Weight:166.133
- Vapor Pressure:9.91E-06mmHg at 25°C
- Melting Point:118 °C
- Boiling Point:372°Cat760mmHg
- Flash Point:172.2°C
- PSA:52.60000
- Density:1.54g/cm3
- LogP:-0.36060
4,10-DIOXATRICYCLO[5.2.1.0(2,6)]DEC-8-ENE-3,5-DIONE(Cas 5426-09-5) Usage
|
General Description |
4,10-DIOXATRICYCLO[5.2.1.0(2,6)]DEC-8-ENE-3,5-DIONE is a chemical compound with a complex and unique ring structure. It contains a tricyclic ring system with two oxygen atoms and a double bond, as well as a ketone functional group. 4,10-DIOXATRICYCLO[5.2.1.0(2,6)]DEC-8-ENE-3,5-DIONE has potential applications in organic synthesis and medicinal chemistry due to its interesting structure and reactivity. Its properties and potential uses make it an interesting target for further research and exploration in the field of organic chemistry. |
InChI:InChI=1/C8H6O4/c9-7-5-3-1-2-4(11-3)6(5)8(10)12-7/h1-6H
5426-09-5 Relevant articles
Organoseleno cytostatic derivatives: Autophagic cell death with AMPK and JNK activation
Garnica, Pablo,Encío, Ignacio,Plano, Daniel,Palop, Juan A.,Sanmartín, Carmen
, p. 234 - 246 (2019)
Selenocyanates and diselenides are poten...
New polymer systems based on alicyclic polyimides
Zhubanov,Kravtsova,Mukhamedova,Bekmagambetova
, p. 1869 - 1874 (2006)
Specific features of modification of som...
Synthesis, crystal structure, spectroscopic properties and potential anti-cancerous activities of four unsaturated bis-norcantharimides
Cheng, Shuang-Shuang,Shi, Yan,Ma, Xiao-Na,Xing, Dian-Xiang,Liu, Lian-Dong,Liu, Yun,Zhao, Yun-Xue,Sui, Qi-Cheng,Tan, Xue-Jie
, p. 228 - 240 (2016)
Four unsaturated norcantharimide (UNCI) ...
New Dielectric Elastomers with Variable Moduli
Hu, Wei,Ren, Zhi,Li, Junpeng,Askounis, Erin,Xie, Zhixin,Pei, Qibing
, p. 4827 - 4836 (2015)
Dielectric elastomers have been widely i...
ACCELERATION OF A DIELS-ALDER REACTION IN AN ULTRACENTRIFUGE
Dolata, Daniel P.,Bergman, Rolf
, p. 707 - 708 (1987)
The reaction rate for the Diels-Alder re...
Synthesis and reactivity of a fluorinated N-alkylmaleimide towards free-radical grafting and polymerization reactions
Castelvetro, Valter,Aglietto, Mauro,Ciardelli, Francesco,Spagnoli, Federica
, p. 315 - 328 (2004)
A new fluorinated N-alkylmaleimide, N-(2...
Albumin-micelles via a one-pot technology platform for the delivery of drugs
Jiang, Yanyan,Liang, Mingtao,Svejkar, Domenic,Hart-Smith, Gene,Lu, Hongxu,Scarano, Wei,Stenzel, Martina H.
, p. 6394 - 6397 (2014)
A new micelle delivery platform based on...
Reversible-Addition Fragmentation Chain Transfer Step-Growth Polymerization
Archer, Noel Edward,Grant, Michael Jeffery,Tanaka, Joji,You, Wei
supporting information, p. 15918 - 15923 (2021/10/21)
Reversible-addition fragmentation chain ...
Bridging from the Sequence to Architecture: Graft Copolymers Engineering via Successive Latent Monomer and Grafting-from Strategies?
Zhang, Yajie,Cao, Xiaohuan,Gao, Yang,Xie, Yujie,Huang, Zhihao,Zhang, Zhengbiao,Zhu, Xiulin
supporting information, p. 1273 - 1280 (2021/05/04)
The on-demand building copolymer structu...
A Mechanochemical Reaction Cascade for Controlling Load-Strengthening of a Mechanochromic Polymer
Boulatov, Roman,Pan, Yifei,Tian, Yancong,Wang, Chenxu,Weng, Wengui,Xiang, Shishuai,Xu, Piaoxue,Zhang, Huan
supporting information, p. 21980 - 21985 (2020/10/02)
We demonstrate an intermolecular reactio...
Method for preparing 4, 10-dioxabicyclo[5.2. 1.0(2, 6)]decane-8-ene-3-ketone
-
Paragraph 0023-0025, (2020/05/30)
The invention discloses a method for pre...
5426-09-5 Process route
-
-
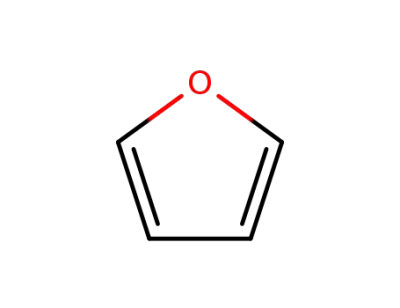
110-00-9
furan

-
-
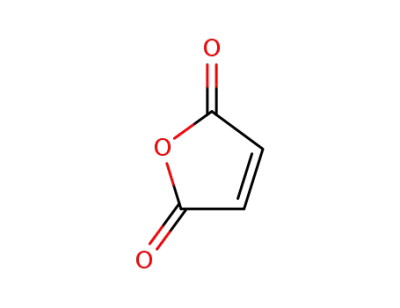
108-31-6
maleic anhydride

-
-
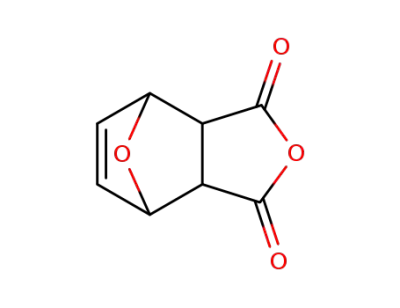
5426-09-5
7-oxanorborn-5-ene-2,3-dicarboxylic anhydride
| Conditions | Yield |
|---|---|
|
In
diethyl ether;
|
100% |
|
In
diethyl ether;
at 20 ℃;
for 6h;
|
98% |
|
In
toluene;
at 20 ℃;
for 72h;
|
97% |
|
In
chloroform;
at 70 ℃;
for 2h;
Temperature;
Solvent;
|
96% |
|
In
benzene;
at 20 ℃;
for 2h;
|
94% |
|
In
diethyl ether;
at 20 ℃;
for 48h;
|
94% |
|
In
toluene;
at 80 ℃;
for 8h;
|
93% |
|
In
tetrahydrofuran;
at 20 ℃;
for 1h;
|
88.4% |
|
In
toluene;
at 80 ℃;
for 6h;
|
87% |
|
In
toluene;
at 80 ℃;
for 6h;
|
87% |
|
In
diethyl ether;
at 25 ℃;
|
87.2% |
|
In
ethyl acetate;
at 35 ℃;
for 24h;
|
87% |
|
In
toluene;
at 20 - 80 ℃;
for 25h;
|
86% |
|
In
toluene;
at 20 ℃;
for 24h;
|
85.6% |
|
In
diethyl ether;
at 38 ℃;
for 1h;
|
85.75% |
|
In
ethyl acetate;
at 38 ℃;
for 1h;
|
85.5% |
|
In
diethyl ether;
at 38 ℃;
for 25h;
|
85.8% |
|
In
diethyl ether;
at 38 ℃;
for 25h;
|
85.8% |
|
In
diethyl ether;
at 38 ℃;
for 1h;
|
85.75% |
|
In
diethyl ether;
at 38 ℃;
for 25h;
|
85.75% |
|
In
toluene;
at 80 ℃;
for 6h;
|
85.1% |
|
In
diethyl ether;
at 38 ℃;
for 25h;
|
85.75% |
|
In
diethyl ether;
at 38 ℃;
for 24h;
|
85.75% |
|
In
diethyl ether;
at 38 ℃;
for 24h;
|
85.75% |
|
In
diethyl ether;
at 20 ℃;
for 41h;
|
84.4% |
|
In
acetone;
at 20 ℃;
for 4h;
Temperature;
|
83.1% |
|
In
diethyl ether;
at 20 ℃;
for 48h;
|
82% |
|
In
toluene;
at 80 ℃;
for 6h;
|
82% |
|
In
benzene;
for 72h;
|
81.4% |
|
In
benzene;
at 20 ℃;
|
80% |
|
In
toluene;
at 20 ℃;
for 24h;
Inert atmosphere;
|
80% |
|
In
toluene;
for 18h;
Reflux;
|
79% |
|
In
tert-butyl methyl ether;
at 45 ℃;
for 0.25h;
Temperature;
Solvent;
|
79.3% |
|
In
toluene;
at 20 ℃;
for 16h;
|
77.4% |
|
In
toluene;
at 20 ℃;
for 16h;
|
77.4% |
|
In
toluene;
at 80 ℃;
|
74% |
|
In
diethyl ether; toluene;
at 80 ℃;
|
74% |
|
In
diethyl ether;
|
64% |
|
In
toluene;
at 20 ℃;
for 36h;
|
54% |
|
In
diethyl ether;
at 20 ℃;
for 24h;
|
48% |
|
In
acetone;
at 20 - 25 ℃;
for 23h;
under 760 Torr;
Product distribution;
comparison between atmospheric pressure and ultracentrifuge (40000g);
|
|
|
In
acetone;
for 23h;
Yield given;
Ambient temperature;
in ultracentrifuge (400000g) or at atmospheric pressure;
|
|
|
Diels Alder synthesis;
|
|
|
|
|
|
at 55 ℃;
for 2h;
under 1639.29 Torr;
Product distribution / selectivity;
|
|
|
at 20 ℃;
for 16h;
Product distribution / selectivity;
|
|
|
In
toluene;
at 20 ℃;
for 48h;
|
|
|
In
acetone; toluene;
at 20 ℃;
|
|
|
In
neat (no solvent);
at 15 - 20 ℃;
for 3h;
|
|
|
With
solid-supported Lewis acid catalyst;
at 80 ℃;
|
|
|
In
toluene;
at 20 ℃;
for 24h;
|
|
|
In
toluene;
for 24h;
Reflux;
|
|
|
|

-

-
5426-09-5
7-oxanorborn-5-ene-2,3-dicarboxylic anhydride
| Conditions | Yield |
|---|---|
|
Furan, Maleinsaeureanhydrid;
|
5426-09-5 Upstream products
-
110-00-9

furan
-
108-31-6

maleic anhydride
5426-09-5 Downstream products
-
49807-46-7
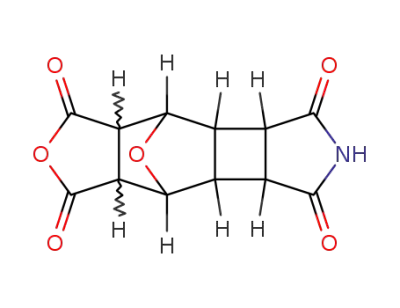
1,3-dioxo-decahydro-4,7-epioxido-benzo[3,4]cyclobuta[1,2-c]pyrrole-5,6-dicarboxylic acid anhydride
-
92789-63-4
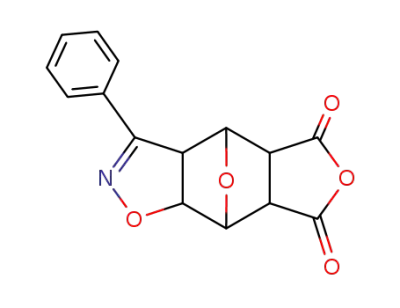
3-phenyl-(3aξ,7aξ)-3a,4,5,6,7,7a-hexahydro-4r,7c-epioxido-benzo[d]isoxazole-5c,6c-dicarboxylic acid anhydride
-
62814-81-7
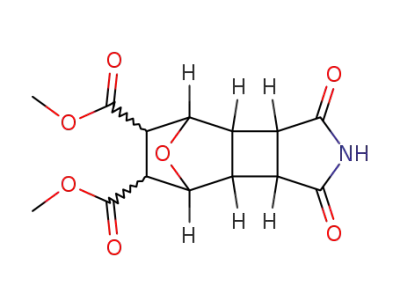
1,3-dioxo-decahydro-4,7-epioxido-benzo[3,4]cyclobuta[1,2-c]pyrrole-5,6-dicarboxylic acid dimethyl ester
-
1164475-59-5
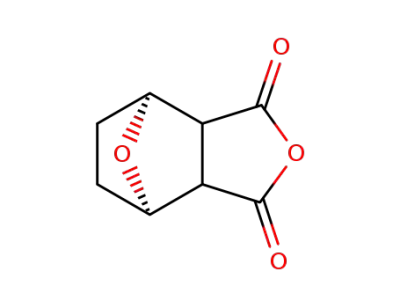
7-oxabicyclo[2,2,1]heptane-2,3-dicarboxylic acid anhydride
Relevant Products
-
DIBASIC ESTER
CAS:95481-62-2
-
Cyclopentane-1,2-dicarboxylic acid anhydride
CAS:35878-28-5
-
Tri-tert-butylphosphine
CAS:13716-12-6