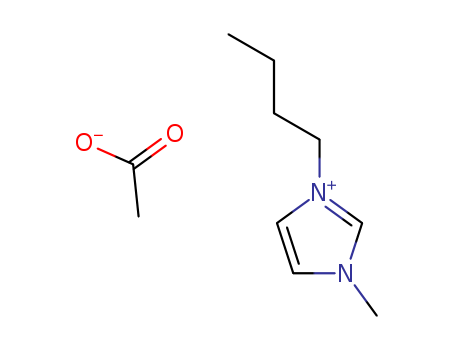
284049-75-8
- Product Name:1-Butyl-3-MethylImidazolium Acetate
- Molecular Formula:C10H18N2O2
- Purity:99%
- Molecular Weight:198.265
Product Details;
CasNo: 284049-75-8
Molecular Formula: C10H18N2O2
factory and manufacture 284049-75-8 1-Butyl-3-MethylImidazolium Acetate lonic liquid
- Molecular Formula:C10H18N2O2
- Molecular Weight:198.265
- Melting Point:-20 °C
- Flash Point:153 °C
- PSA:48.94000
- Density:1.05g/ml
- LogP:-0.13110
1-BUTYL-3-METHYLIMIDAZOLIUM ACETATE(Cas 284049-75-8) Usage
|
Conductivity |
1.44 mS/cm (30 °C) |
|
General Description |
1-Butyl-3-methylimidazolium acetate (BMIM Acetate) is an ionic liquid known for its effectiveness as a solvent for cellulose and chitosan dissolution. Its physicochemical properties, such as viscosity and conductivity, are influenced by temperature and van der Waals interactions, with longer alkyl chains in carboxylate anions increasing ion pairing and reducing conductivity. Additionally, it exhibits salting-out effects in aqueous two-phase systems and can dissolve cellulose at ambient temperatures when combined with polar cosolvents. The anion's alkyl chain length also impacts cellulose solubility, with shorter chains generally enhancing dissolution. These properties make it valuable for applications in polymer processing, recycling, and green chemistry. |
InChI:InChI=1/C8H15N2.C2H4O2/c1-3-4-5-10-7-6-9(2)8-10;1-2(3)4/h6-8H,3-5H2,1-2H3;1H3,(H,3,4)/q+1;/p-1
284049-75-8 Relevant articles
Viscosities and conductivities of 1-butyl-3-methylimidazolium carboxylates ionic liquids at different temperatures
Xu, Airong,Zhang, Yajuan,Li, Zhiyong,Wang, Jianji
, p. 3102 - 3108 (2012)
In recent years, 1-butyl-3-methylimidazo...
On the Mechanism of the Reactivity of 1,3-Dialkylimidazolium Salts under Basic to Acidic Conditions: A Combined Kinetic and Computational Study
Rico del Cerro, Daniel,Mera-Adasme, Raúl,King, Alistair W. T.,Perea-Buceta, Jesus E.,Heikkinen, Sami,Hase, Tapio,Sundholm, Dage,W?h?l?, Kristiina
, p. 11613 - 11617 (2018)
Comprehensive spectroscopic kinetic stud...
Salting-out effect of ionic liquids on poly(propylene glycol) (PPG): Formation of PPG + ionic liquid aqueous two-phase systems
Wu, Changzeng,Wang, Jianji,Pei, Yuanchao,Wang, Huiyong,Li, Zhiyong
, p. 5004 - 5008 (2010)
In the present work, aqueous poly(propyl...
Cellulose dissolution at ambient temperature: Role of preferential solvation of cations of ionic liquids by a cosolvent
Xu, Airong,Zhang, Yajuan,Zhao, Yang,Wang, Jianji
, p. 540 - 544 (2013)
Highly effective cellulose solvents for ...
Effect of alkyl chain length in anion on dissolution of cellulose in 1-butyl-3-methylimidazolium carboxylate ionic liquids
Xu, Airong,Zhang, Yibo,Lu, Weiwei,Yao, Kaisheng,Xu, Hang
, p. 211 - 214 (2014)
In recent years, 1-butyl-3-methylimidazo...
The effect of the ionic liquid anion in the pretreatment of pine wood chips
Brandt, Agnieszka,Hallett, Jason P.,Leak, David J.,Murphy, Richard J.,Welton, Tom
, p. 672 - 679 (2010)
The effect of the anion of ionic liquids...
Effects of anionic structure and lithium salts addition on the dissolution of cellulose in 1-butyl-3-methylimidazolium-based ionic liquid solvent systems
Xu, Airong,Wang, Jianji,Wang, Huiyong
, p. 268 - 275 (2010)
Cellulose is the most abundant biorenewa...
Rhodium nanoparticles impregnated on TiO2: Strong morphological effects on hydrogen production
Albuquerque, Brunno L.,Chacón, Gustavo,Nazarkovsky, Michael,Dupont, Jairton
, p. 13249 - 13258 (2020)
The effect of the shape of rhodium nanop...
Interactions of 1-butyl-3-methylimidazolium carboxylate ionic liquids with glucose in water: A study of volumetric properties, viscosity, conductivity and NMR
Zhuo, Kelei,Chen, Yujuan,Chen, Jing,Bai, Guangyue,Wang, Jianji
, p. 14542 - 14549 (2011)
Extensive applications of ionic liquids ...
Experimental and theoretical studies on solvation in aqueous solutions of ionic liquids carrying different side chains: the n-butyl-group versus the methoxyethyl group
De Jesus, Jessica C.,Pires, Paulo A. R.,Mustafa, Rizwana,Riaz, Naheed,El Seoud, Omar A.
, p. 15952 - 15963 (2017)
We used solvatochromic compounds to prob...
Binary mixtures of ionic liquids-DMSO as solvents for the dissolution and derivatization of cellulose: Effects of alkyl and alkoxy side chains
Ferreira, Daniela C.,Oliveira, Mayara L.,Bioni, Thais A.,Nawaz, Haq,King, Alistair W.T.,Kilpel?inen, Ilkka,Hummel, Michael,Sixta, Herbert,El Seoud, Omar A.
, p. 206 - 214 (2019)
The efficiency of mixtures of ionic liqu...
Water sorption by ionic liquids: Evidence of a diffusion-controlled sorption process derived from the case study of [BMIm][OAc]
Spie?, Alex,Brandt, Philipp,Schmitz, Alexa,Janiak, Christoph
, (2021/11/24)
The hydrophilicity of ionic liquids (ILs...
Electrochemical and spectroscopic study of vanadyl acetylacetonate–ionic liquids interactions
Guglielmero,Langroudi, Mo. Meskinfam,Khatib, M. Al,de Oliveira, M. Aysla Costa,Mecheri,De Leo,Mezzetta,Guazzelli,Giglioli,Epifanio, A. D',Pogni,Chiappe,Pomelli
, (2021/02/16)
A panel of ionic liquids has been synthe...
Double-water-phase ionic liquid synthesis method
-
Paragraph 0041-0043, (2020/11/23)
The invention belongs to the field of ch...
Synthesis of hydrotalcites from waste steel slag with [Bmim]OH intercalated for the transesterification of glycerol carbonate
Liu, Guanhao,Xu, Xinru,Yang, Jingyi
, (2020/10/12)
Ca-Mg-Al hydrotalcites were prepared by ...
284049-75-8 Process route
-
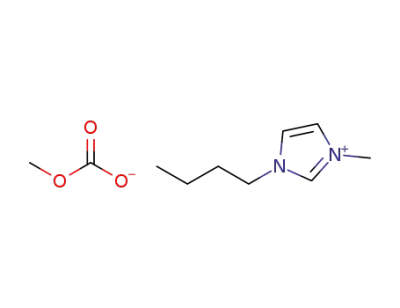
-
916850-37-8
1-butyl-3-methyl-1H-imidazol-3-ium methylcarbonate

-
-
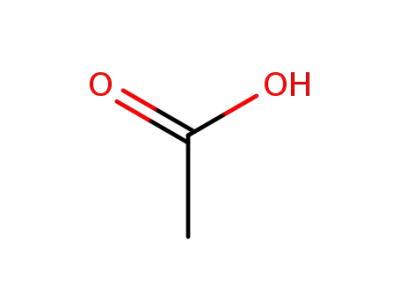
64-19-7,77671-22-8
acetic acid

-
-
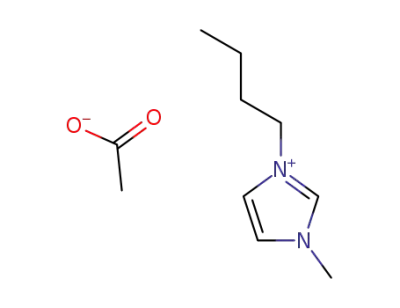
284049-75-8
3-butyl-1-methylimidazolium acetate
| Conditions | Yield |
|---|---|
|
In
methanol;
at 0 - 20 ℃;
for 1h;
|
100% |
|
In
methanol;
for 1h;
Schlenk technique;
|
|
|
In
methanol;
|
|
|
In
methanol;
at 20 - 50 ℃;
for 2h;
|
-
-
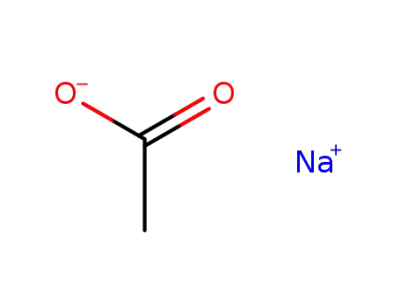
127-09-3
sodium acetate

-
-
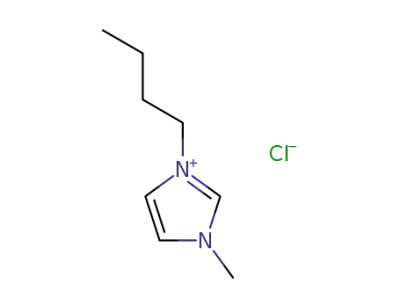
79917-90-1
1-butyl-3-methylimidazolium chloride

-

-
284049-75-8
3-butyl-1-methylimidazolium acetate
| Conditions | Yield |
|---|---|
|
With
L-lysine;
In
water;
at 25 ℃;
for 0.5h;
|
58% |
|
In
ethanol;
for 24h;
|
|
|
In
ethanol;
at 39.84 ℃;
for 12h;
|
284049-75-8 Upstream products
-
127-09-3

sodium acetate
-
4316-42-1
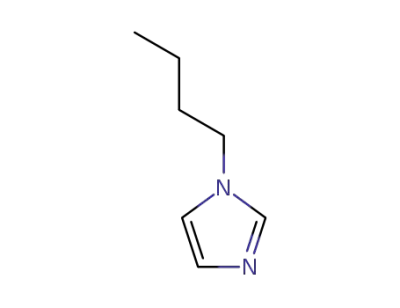
1-Butylimidazole
-
616-42-2
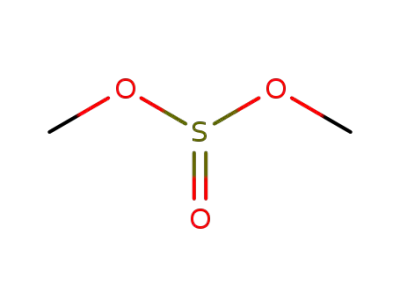
dimethylsulfite
-
64-19-7

acetic acid
284049-75-8 Downstream products
-
174899-94-6
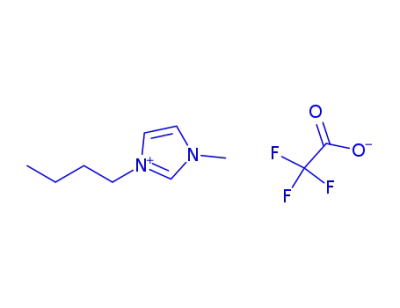
1-butyl-3-methylimidazolium trifluoroacetate
-
342789-81-5
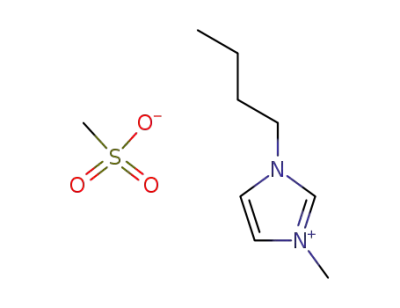
1-n-butyl-3-methylimidazolium methanesulfonate
-
79917-90-1

1-butyl-3-methylimidazolium chloride
-
112-14-1
n-octyl acetate
Relevant Products
-
2,5-Dimethoxy-Beta-Nitrostyrene
CAS:40276-11-7
-
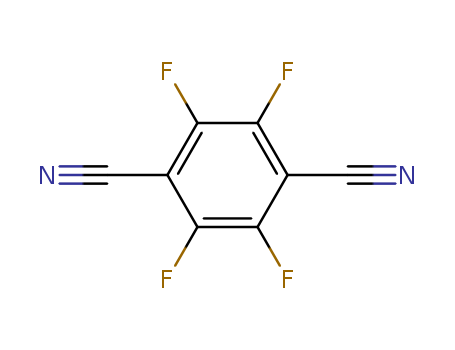
Tetrafluoroterephthalonitrile
CAS:1835-49-0
-
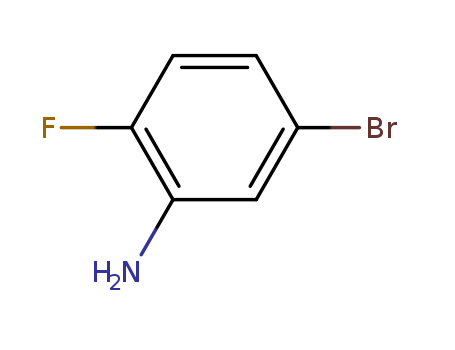
5-Bromo-2-fluoroaniline
CAS:2924-09-6