443-88-9
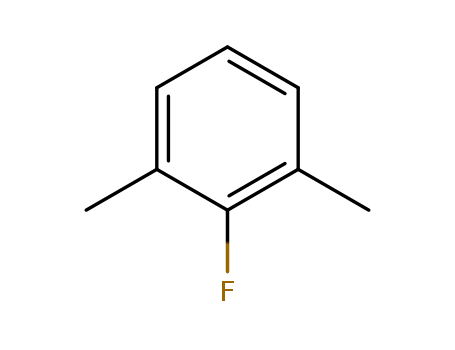
- Product Name:2,6-Dimethylfluorobenzene
- Molecular Formula:C8H9F
- Purity:99%
- Molecular Weight:124.158
Product Details;
CasNo: 443-88-9
Molecular Formula: C8H9F
factory and supplier 443-88-9 2,6-Dimethylfluorobenzene in stock
- Molecular Formula:C8H9F
- Molecular Weight:124.158
- Vapor Pressure:6.56mmHg at 25°C
- Refractive Index:n20/D 1.479(lit.)
- Boiling Point:143.9 °C at 760 mmHg
- Flash Point:30.4 °C
- PSA:0.00000
- Density:0.983 g/cm3
- LogP:2.44250
2,6-Dimethylfluorobenzene(Cas 443-88-9) Usage
|
Synthesis Reference(s) |
Journal of Medicinal Chemistry, 21, p. 906, 1978 DOI: 10.1021/jm00207a013 |
InChI:InChI=1/C8H9F/c1-6-4-3-5-7(2)8(6)9/h3-5H,1-2H3
443-88-9 Relevant articles
Azoacetylenes for the Synthesis of Arylazotriazole Photoswitches
Anderl, Felix,Balkenhohl, Moritz,Carreira, Erick M.,Fink, Moritz,Pfaff, Patrick
, p. 14495 - 14501 (2021/09/18)
We report a modular approach toward nove...
Expanding the Balz–Schiemann Reaction: Organotrifluoroborates Serve as Competent Sources of Fluoride Ion for Fluoro-Dediazoniation
Mohy El Dine, Tharwat,Sadek, Omar,Gras, Emmanuel,Perrin, David M.
supporting information, p. 14933 - 14937 (2018/09/25)
The Balz–Schiemann reaction endures as a...
Fluorination of aryl boronic acids using acetyl hypofluorite made directly from diluted fluorine
Vints, Inna,Gatenyo, Julia,Rozen, Shlomo
, p. 11794 - 11797 (2014/01/06)
Aryl boronic acids or pinacol esters con...
Cu-catalyzed fluorination of diaryliodonium salts with KF
Ichiishi, Naoko,Canty, Allan J.,Yates, Brian F.,Sanford, Melanie S.
supporting information, p. 5134 - 5137 (2013/10/22)
A mild Cu-catalyzed nucleophilic fluorin...
443-88-9 Process route
-
-
87-62-7
2,6-dimethylaniline

-
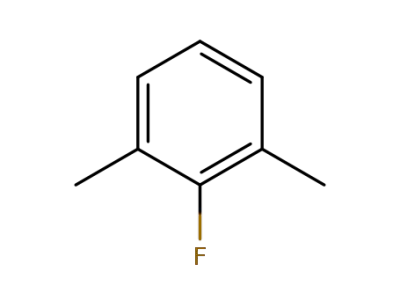
-
443-88-9
2-fluoro-m-xylene
| Conditions | Yield |
|---|---|
|
With
sodium nitrite;
In
Et3N-3HF; diethyl ether;
|
86.3% |
|
With
hydrogenchloride; sodium tetrafluoroborate; sodium nitrite;
In
water;
at 0 - 5 ℃;
|
41% |
|
|
|
|
Multistep reaction;
(i) NaNO2, aq. HBF4, THF, (ii) benzene;
|
|
|
Multi-step reaction with 2 steps
1: 60 percent / 37percent aq.HCl, NaNO2, HBF4 / 0.5 h / 1.) 10 deg C, 2.) 0 - 10 deg C, 10 min.
2: 53 percent / 0.5 h / Heating
With
hydrogenchloride; tetrafluoroboric acid; sodium nitrite;
|
|
|
|
|
|
2,6-dimethylaniline;
With
phosphoric acid;
In
tert-butyl alcohol;
Inert atmosphere;
With
tert.-butylnitrite; potassium phenyltrifluoborate;
In
tert-butyl alcohol;
at 20 - 50 ℃;
for 2.33333h;
Inert atmosphere;
With
2,4-Dinitrofluorobenzene;
In
dimethylsulfoxide-d6; tert-butyl alcohol;
at 20 ℃;
Inert atmosphere;
|
50 %Spectr. |
|
Multi-step reaction with 2 steps
1: ethyl acetate / 0 °C / Inert atmosphere
2: [D3]acetonitrile
In
[D3]acetonitrile; ethyl acetate;
|
-
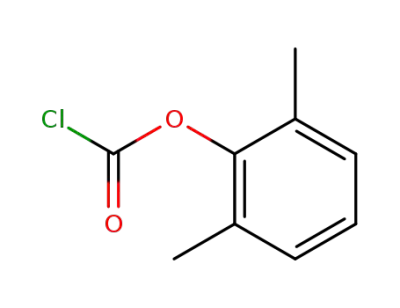
-
876-99-3
2,6-dimethylphenyl chloroformic acid ester

-

-
443-88-9
2-fluoro-m-xylene
| Conditions | Yield |
|---|---|
|
With
hydrogen fluoride;
In
various solvent(s);
at 140 ℃;
for 6h;
under 19501.6 Torr;
|
90% |
|
Multi-step reaction with 2 steps
1: 80 percent / potassium fluoride, 18-crown-6 / CH2Cl2 / 5 h / other reagent: hydrogen fluoride
2: 40 percent / SbF5 / 180 h / Heating
With
potassium fluoride; 18-crown-6 ether; antimony pentafluoride;
In
dichloromethane;
|
443-88-9 Upstream products
-
87-62-7

2,6-dimethylaniline
-
2366-75-8
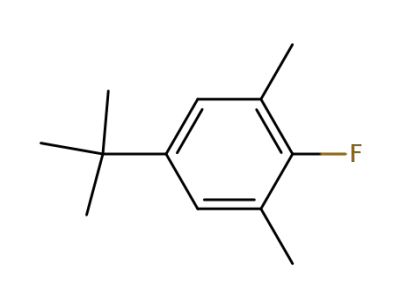
5-tert-butyl-2-fluoro-1,3-dimethylbenzene
-
108-38-3
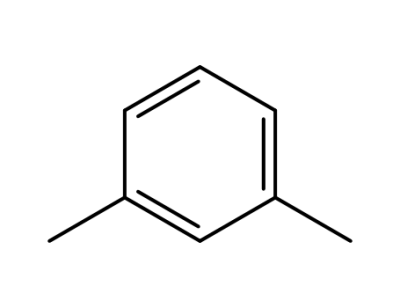
m-xylene
-
2192-33-8
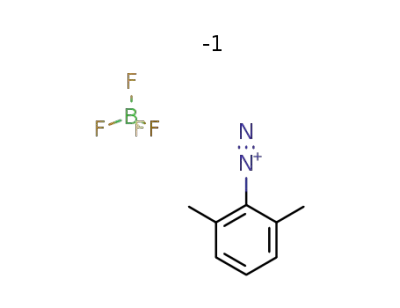
2,6-dimethylbenzenediazonium tetrafluoroborate
443-88-9 Downstream products
-
25006-86-4
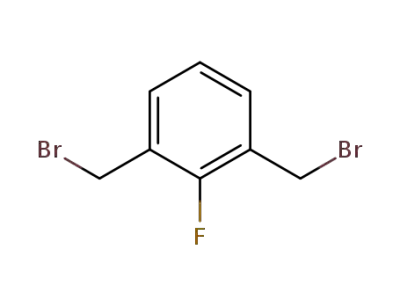
2,6-bis(bromomethyl)-1-fluorobenzene
-
67205-30-5
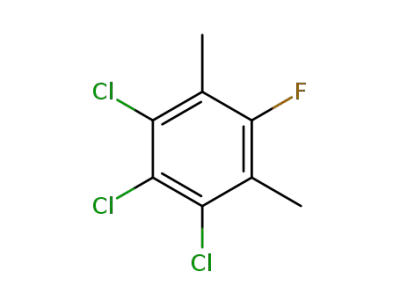
1,2,3-Trichloro-5-fluoro-4,6-dimethyl-benzene
-
106776-16-3
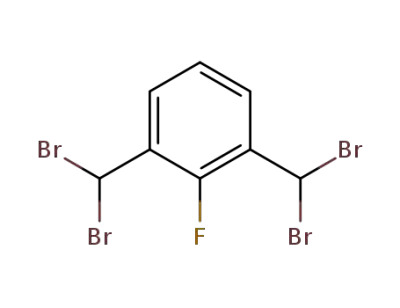
1,3-Bis(dibromomethyl)-2-fluorobenzene
-
1583-65-9
2-fluoro-isophthalic acid
Relevant Products
-
5-Bromo-2-fluoro-m-xylene
CAS:99725-44-7
-
3,6-Dibromopyridazide
CAS:17973-86-3
-
1-Butyl-3-methylimidazolium tetrafluoroborate
CAS:174501-65-6