440-60-8
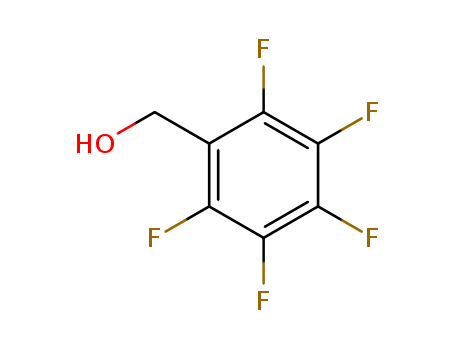
- Product Name:2,3,4,5,6-Pentafluorobenzyl alcohol
- Molecular Formula:C7H3F5O
- Purity:99%
- Molecular Weight:198.092
Product Details;
CasNo: 440-60-8
Molecular Formula: C7H3F5O
Appearance: white crystalline mass
factory and supplier 440-60-8 2,3,4,5,6-Pentafluorobenzyl alcohol in stock
- Molecular Formula:C7H3F5O
- Molecular Weight:198.092
- Appearance/Colour:white crystalline mass
- Vapor Pressure:0.115mmHg at 25°C
- Melting Point:37-38 °C(lit.)
- Refractive Index:1.487
- Boiling Point:181 °C at 760 mmHg
- PKA:13.07±0.10(Predicted)
- Flash Point:87.8 °C
- PSA:20.23000
- Density:1.593 g/cm3
- LogP:1.87440
2,3,4,5,6-Pentafluorobenzyl alcohol(Cas 440-60-8) Usage
|
Definition |
ChEBI: An organofluorine compound that is benzyl alcohol substituted by fluoro groups at positions 2, 3, 4, 5 and 6. |
|
General Description |
Structures of complex of horse liver alchohol dehydrogenase enzyme with 2,3,4,5,6-pentafluorobenzyl alcohol has been investigated by X-ray crystallography. |
InChI:InChI=1/C9H9FO2/c1-2-12-9(11)7-5-3-4-6-8(7)10/h3-6H,2H2,1H3
440-60-8 Relevant articles
Mono- and Di-Mesoionic Carbene-Boranes: Synthesis, Structures and Utility as Reducing Agents
Stein, Felix,Kirsch, Marius,Beerhues, Julia,Albold, Uta,Sarkar, Biprajit
supporting information, p. 2417 - 2424 (2021/06/17)
Mesoionic carbenes (MIC) of the 1,2,3-tr...
Chemoselective and Site-Selective Reductions Catalyzed by a Supramolecular Host and a Pyridine-Borane Cofactor
Morimoto, Mariko,Cao, Wendy,Bergman, Robert G.,Raymond, Kenneth N.,Toste, F. Dean
supporting information, p. 2108 - 2114 (2021/02/06)
Supramolecular catalysts emulate the mec...
Copper(II)-Catalyzed Selective Hydroboration of Ketones and Aldehydes
Zeng, Haisu,Wu, Jing,Li, Sihan,Hui, Christina,Ta, Anita,Cheng, Shu-Yuan,Zheng, Shengping,Zhang, Guoqi
, p. 401 - 406 (2019/01/23)
A novel nonanuclear copper(II) complex o...
Method for preparing polyfluorobenzyl alcohol
-
Paragraph 0039; 0040, (2018/05/16)
The invention relates to a method for pr...
440-60-8 Process route
-

-
653-37-2
perfluorobenzaldehyde

-

-
440-60-8
(2,3,4,5,6-pentafluorophenyl)methanol
| Conditions | Yield |
|---|---|
|
perfluorobenzaldehyde;
With
C24H20Cl2F5NRuS; isopropyl alcohol;
at 82 ℃;
for 0.166667h;
With
potassium hydroxide;
at 82 ℃;
for 0.5h;
|
100% |
|
With
sodium tetrahydroborate; 1,4,8,11,15,18,22,25-octapentyloxyphthalocyaninato nickel;
In
pentan-1-ol;
at 25 ℃;
for 0.416667h;
Catalytic behavior;
|
87% |
|
With
tris(pentafluorophenyl)borate; hydrogen;
In
1,4-dioxane;
at 80 ℃;
for 24h;
under 3750.38 Torr;
Glovebox;
Inert atmosphere;
|
74% |
|
With
glucose dehydrogenase; D-glucose; Bacteroides fragilis ATCC 25285 7α-hydroxysteroid dehydrogenase; NAD;
In
dimethyl sulfoxide;
at 30 ℃;
for 42h;
pH=7;
aq. phosphate buffer;
Enzymatic reaction;
|
72% |
|
With
lithium aluminium tetrahydride;
|
|
|
With
Dimethylphenylsilane; o-phenylenebis(diphenylphosphine); potassium tert-butylate; copper(l) chloride;
In
tetrahydrofuran;
for 12h;
regioselective reaction;
Inert atmosphere;
Reflux;
|
|
|
With
D-glucose; D-glucose dehydrogenase; a putative aldehyde reductase (OsAR) from Oceanospirillum sp.MED92; NADPH;
In
aq. phosphate buffer;
at 25 ℃;
for 18h;
pH=6.5;
chemoselective reaction;
Enzymatic reaction;
|
|
|
Multi-step reaction with 2 steps
1: C27H44AlN3 / 0.33 h / 20 °C / Inert atmosphere; Glovebox
2: silica gel / Inert atmosphere; Heating
With
C27H44AlN3; silica gel;
|
|
|
With
C40H46ClN2OPRu(2+)*F6P(1-)*Cl(1-); sodium formate;
In
water;
at 80 ℃;
for 5h;
Catalytic behavior;
Inert atmosphere;
Schlenk technique;
|
97 %Chromat. |
|
Multi-step reaction with 2 steps
1: C74H94Cu9N4O28 / neat (no solvent) / 1 h / 20 °C / Glovebox; Inert atmosphere
2: silica gel / ethyl acetate; hexane / 20 °C
With
C74H94Cu9N4O28; silica gel;
In
hexane; ethyl acetate;
|
|
|
With
C21H28BN3; silica gel;
In
dichloromethane;
at 20 ℃;
for 3h;
Inert atmosphere;
Schlenk technique;
|
35 %Spectr. |
-

-
15989-99-8
2,3,4,5,6-pentafluorobenzoic anhydride

-

-
440-60-8
(2,3,4,5,6-pentafluorophenyl)methanol
| Conditions | Yield |
|---|---|
|
With
sodium tetrahydroborate;
In
ethanol;
for 1h;
|
98% |
440-60-8 Upstream products
-
1548-77-2

2,3,4,5,6-pentafluorobenzylamine
-
67-56-1

methanol
-
392-56-3

Hexafluorobenzene
-
66820-45-9

1-hydroxymethyl-1,2,3,4,5,6-hexafluoro-3,5-cyclohexadiene
440-60-8 Downstream products
-
53526-74-2

2,3,4,5,6-pentafluorobenzyl chloroformate
-
121720-32-9

C22H17F5O2
-
121720-32-9

C22H17F5O2
-
121720-32-9

C22H17F5O2
Relevant Products
-
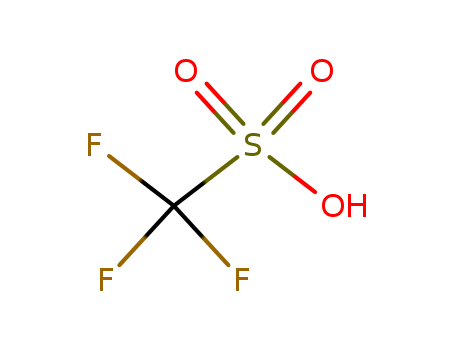
Trifluoromethanesulfonic acid
CAS:1493-13-6
-
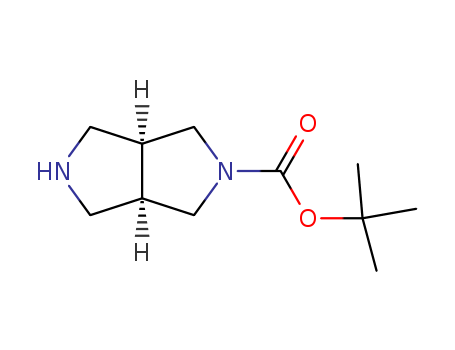
Cis-2-Boc-hexahydropyrrolo(3,4-c)pyrrole
CAS:250275-15-1
-
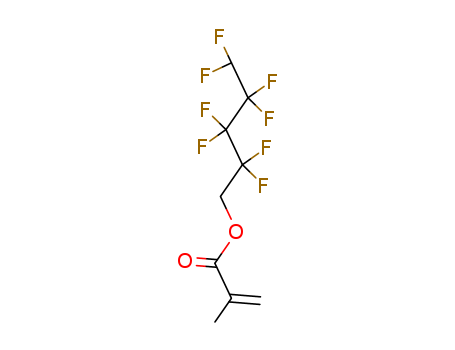
1H,1H,5H-Octafluoropentyl Methacrylate
CAS:355-93-1