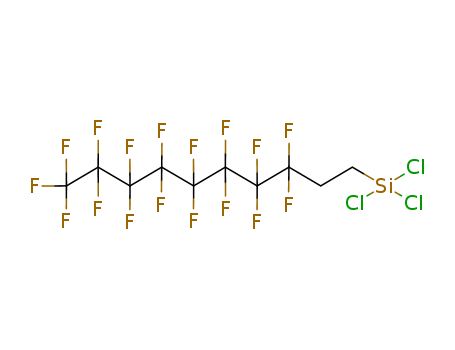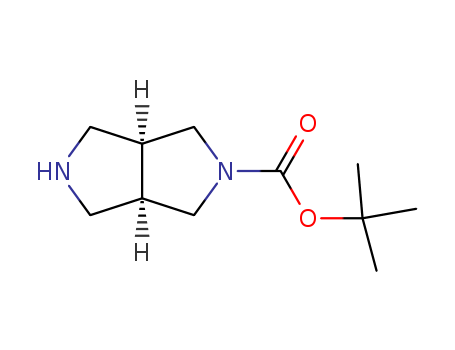
250275-15-1
- Product Name:Cis-2-Boc-hexahydropyrrolo(3,4-c)pyrrole
- Molecular Formula:C11H20N2O2
- Purity:99%
- Molecular Weight:212.292
Product Details;
CasNo: 250275-15-1
Molecular Formula: C11H20N2O2
factory and supplier 250275-15-1 Cis-2-Boc-hexahydropyrrolo(3,4-c)pyrrole in stock
- Molecular Formula:C11H20N2O2
- Molecular Weight:212.292
- Vapor Pressure:0.002mmHg at 25°C
- Refractive Index:1.494
- Boiling Point:295.397 °C at 760 mmHg
- PKA:10.72±0.20(Predicted)
- Flash Point:132.451 °C
- PSA:41.57000
- Density:1.076 g/cm3
- LogP:1.33940
cis-2-Boc-hexahydropyrrolo[3,4-c]pyrrole(Cas 250275-15-1) Usage
|
General Description |
Cis-2-Boc-hexahydropyrrolo[3,4-c]pyrrole is a chemical compound with a unique molecular structure that is used in organic synthesis and medicinal chemistry. It is a bicyclic compound containing a pyrrolopyrrole core and a Boc (tert-butoxycarbonyl) protecting group at the 2-position. cis-2-Boc-hexahydropyrrolo[3,4-c]pyrrole is commonly used as a building block in the synthesis of various biologically active molecules and pharmaceuticals. Its unique structure and reactivity make it a versatile and valuable tool in the field of chemical synthesis and drug discovery. Additionally, its stable nature and relatively simple synthesis route make it an attractive option for researchers and chemists working in the field of drug development and organic chemistry. |
InChI:InChI=1/C11H20N2O2/c1-11(2,3)15-10(14)13-6-8-4-12-5-9(8)7-13/h8-9,12H,4-7H2,1-3H3
250275-15-1 Relevant articles
QUINOLONE DERIVATIVES AS FIBROBLAST GROWTH FACTOR RECEPTOR INHIBITORS
-
Page/Page column 86, (2016/12/16)
Compounds of formula (I) that are Fibrob...
QUINOLONE DERIVATIVES AS FIBROBLAST GROWTH FACTOR RECEPTOR INHIBITORS
-
Page/Page column 139; 140, (2015/09/23)
Compounds that are Fibroblast Growth Fac...
Novel Octahydropyrrolo[3,4- c ]pyrroles Are Selective Orexin-2 Antagonists: SAR Leading to a Clinical Candidate
Letavic, Michael A.,Bonaventure, Pascal,Carruthers, Nicholas I.,Dugovic, Christine,Koudriakova, Tatiana,Lord, Brian,Lovenberg, Timothy W.,Ly, Kiev S.,Mani, Neelakandha S.,Nepomuceno, Diane,Pippel, Daniel J.,Rizzolio, Michele,Shelton, Jonathan E.,Shah, Chandra R.,Shireman, Brock T.,Young, Lana K.,Yun, Sujin
, p. 5620 - 5636 (2015/08/03)
The preclinical characterization of nove...
Selective Ligands for the Neuronal Nicotinic Receptors and Uses Thereof
-
Page/Page column 10, (2009/12/04)
The present application describes select...
250275-15-1 Process route
-
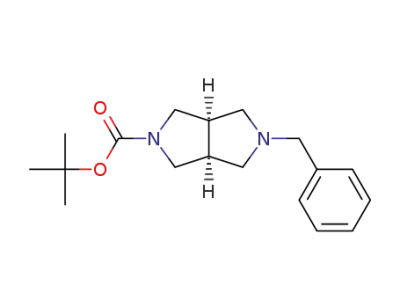
![(3aR,6aS)-tert-butyl 5-benzylhexahydropyrrolo[3,4-c]pyrrole-2(1H)-carboxylate](/upload/2025/12/d4b3ebb8-6769-4fdc-9def-313d714ec74c.png)
-
370879-56-4
(3aR,6aS)-tert-butyl 5-benzylhexahydropyrrolo[3,4-c]pyrrole-2(1H)-carboxylate

-
![tert-butyl (3aR,6aS)-hexahydropyrrolo[3,4-c]pyrrole-2(1H)-carboxylate](/upload/2025/12/0e369b4e-b84d-4477-899e-3a5aa9dc20dc.png)
-
250275-15-1,1256094-14-0
tert-butyl (3aR,6aS)-hexahydropyrrolo[3,4-c]pyrrole-2(1H)-carboxylate
| Conditions | Yield |
|---|---|
|
palladium-carbon;
In
ethanol;
|
88% |
|
With
10 wt% Pd(OH)2 on carbon; hydrogen;
In
methanol;
at 60 ℃;
under 3102.97 Torr;
|
66% |
|
With
10% palladium hydroxide on charcoal; hydrogen;
In
methanol;
at 60 ℃;
under 3102.97 Torr;
|
66% |
|
With
hydrogen;
Pd(OH)2/C;
In
methanol;
at 50 ℃;
for 4h;
under 3102.97 Torr;
|
|
|
With
20% Pd(OH)2/C; hydrogen;
In
methanol;
for 4h;
under 3102.97 Torr;
Inert atmosphere;
|
33.84 g |
|
With
palladium 10% on activated carbon; hydrogen; acetic acid;
In
methanol;
for 72h;
under 3620.13 Torr;
Inert atmosphere;
|
-
![cis-3-benzyl-3,7-diazabicyclo[3.3.0]octane](/upload/2025/12/e6be0367-51e4-4770-8bbb-3debeb3007e9.png)
-
172739-04-7
cis-3-benzyl-3,7-diazabicyclo[3.3.0]octane

-
![tert-butyl (3aR,6aS)-hexahydropyrrolo[3,4-c]pyrrole-2(1H)-carboxylate](/upload/2025/12/0e369b4e-b84d-4477-899e-3a5aa9dc20dc.png)
-
250275-15-1,1256094-14-0
tert-butyl (3aR,6aS)-hexahydropyrrolo[3,4-c]pyrrole-2(1H)-carboxylate
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 2 steps
1: dichloromethane / 24 h / 23 °C / Inert atmosphere
2: hydrogen; acetic acid; palladium 10% on activated carbon / methanol / 72 h / 3620.13 Torr / Inert atmosphere
With
palladium 10% on activated carbon; hydrogen; acetic acid;
In
methanol; dichloromethane;
|
|
|
Multi-step reaction with 2 steps
1: N-ethyl-N,N-diisopropylamine / tetrahydrofuran / 5 h / 20 °C
2: 10 wt% Pd(OH)2 on carbon; hydrogen / methanol / 60 °C / 3102.97 Torr
With
10 wt% Pd(OH)2 on carbon; hydrogen; N-ethyl-N,N-diisopropylamine;
In
tetrahydrofuran; methanol;
|
|
|
Multi-step reaction with 2 steps
1: N-ethyl-N,N-diisopropylamine / tetrahydrofuran / 5 h / 20 °C
2: 10% palladium hydroxide on charcoal; hydrogen / methanol / 60 °C / 3102.97 Torr
With
10% palladium hydroxide on charcoal; hydrogen; N-ethyl-N,N-diisopropylamine;
In
tetrahydrofuran; methanol;
|
250275-15-1 Upstream products
-
370879-56-4
(3aR,6aS)-tert-butyl 5-benzylhexahydropyrrolo[3,4-c]pyrrole-2(1H)-carboxylate
-
172739-04-7
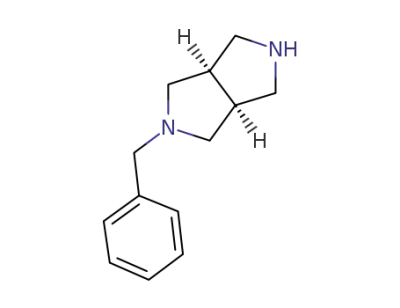
cis-3-benzyl-3,7-diazabicyclo[3.3.0]octane
-
24424-99-5
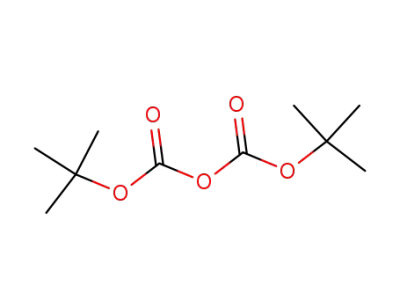
di-tert-butyl dicarbonate
-
370879-53-1
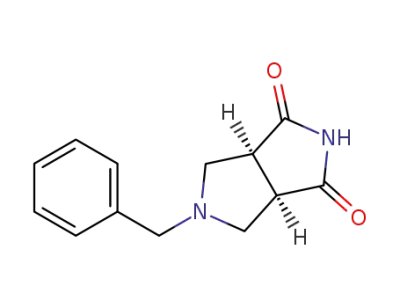
(3aR,6aS)-5-benzyltetrahydropyrrolo[3,4-c]pyrrole-1,3(2H,3aH)-dione
250275-15-1 Downstream products
-
1194688-13-5
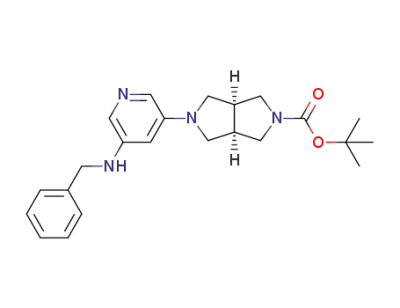
(3aR,6aS)-tert-butyl 5-(5-(benzylamino)pyridin-3-yl)hexahydropyrrolo[3,4-c]pyrrole-2(1H)-carboxylate
-
1194688-41-9
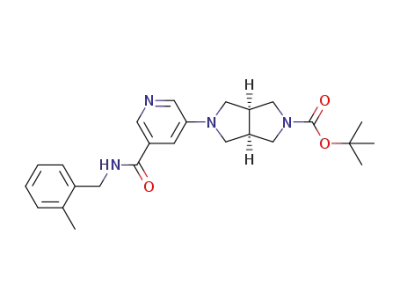
(3aR,6aS)-tert-butyl 5-(5-(2-methylbenzylcarbamoyl)pyridin-3-yl)hexahydropyrrolo[3,4-c]pyrrole-2(1H)-carboxylate
-
1194687-49-4
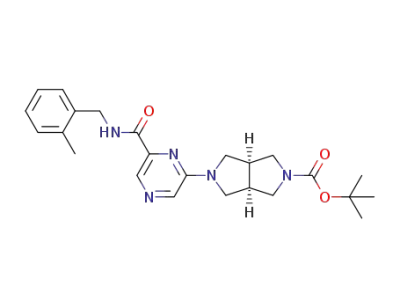
(3aR,6aS)-tert-butyl 5-(6-(2-methylbenzylcarbamoyl)pyrazin-2-yl)hexahydropyrrolo[3,4-c]pyrrole-2(1H)-carboxylate
-
1194687-78-9

(3aR,6aS)-tert-butyl 5-(6-(3-isopropoxyphenylcarbamoyl)pyrazin-2-yl)hexahydropyrrolo[3,4-c]pyrrole-2(1H)-carboxylate
Relevant Products
-
2,5-Dimethoxy-Beta-Nitrostyrene
CAS:40276-11-7
-
1H,1H,2H,2H-Perfluorodecyltrichlorosilane
CAS:78560-44-8
-
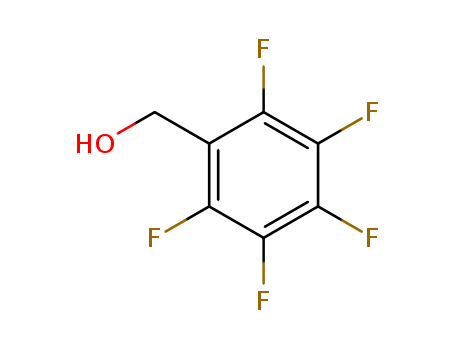
2,3,4,5,6-Pentafluorobenzyl alcohol
CAS:440-60-8