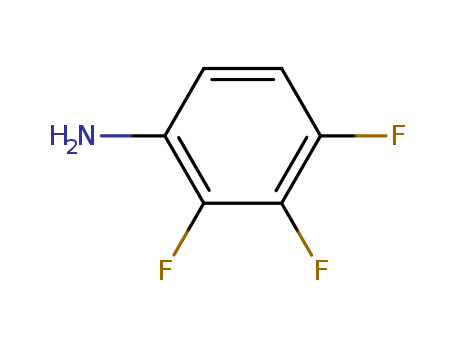
3862-73-5
- Product Name:2,3,4-Trifluorobenzenamine
- Molecular Formula:C6H4F3N
- Purity:99%
- Molecular Weight:147.1
Product Details;
CasNo: 3862-73-5
Molecular Formula: C6H4F3N
Appearance: Pale yellow liquid
factory and supplier 3862-73-5 2,3,4-Trifluorobenzenamine in stock
- Molecular Formula:C6H4F3N
- Molecular Weight:147.1
- Appearance/Colour:Pale yellow liquid
- Vapor Pressure:0.00696mmHg at 25°C
- Melting Point:14-15°C
- Refractive Index:n20/D 1.486(lit.)
- Boiling Point:173.8 °C at 760 mmHg
- PKA:2.25±0.10(Predicted)
- Flash Point:71.8 °C
- PSA:26.02000
- Density:1.409 g/cm3
- LogP:2.26730
2,3,4-Trifluorobenzenamine(Cas 3862-73-5) Usage
|
Safety Profile |
Moderately toxic by ingestion. Askin and eye irritant. Combustible liquid. When heated todecomposition it emits toxic fumes of NOx and F??. |
|
General Description |
Study on synthesis of 2,3,4-trifluoroaniline was reported. |
InChI:InChI=1/C7H5F3N2O/c8-7(9,10)5-2-1-4(3-12-5)6(11)13/h1-3H,(H2,11,13)
3862-73-5 Relevant articles
Pd-CATALYZED AMINATION OF FLUORINATED ARYL CHLORIDES
-
Page/Page column 12; 13, (2021/10/15)
The presently claimed invention relates ...
Preparation method of 3, 4, 5-trifluorobromobenzene
-
, (2020/12/14)
The invention relates to a preparation m...
Highly selective hydrogenation of halogenated nitroarenes over Ru/CN nanocomposites by: In situ pyrolysis
Yue, Shengnan,Wang, Xueguang,Li, Shaoting,Sheng, Yao,Zou, Xiujing,Lu, Xionggang,Zhang, Chunlei
, p. 11861 - 11869 (2020/07/28)
A highly chemoselective and recyclable r...
Method for producing 2,3,4-trifluoroaniline by adopting solvent-free catalytic hydrogenation
-
Paragraph 0018-0033, (2019/01/06)
The invention provides a method for prod...
3862-73-5 Process route
-
-
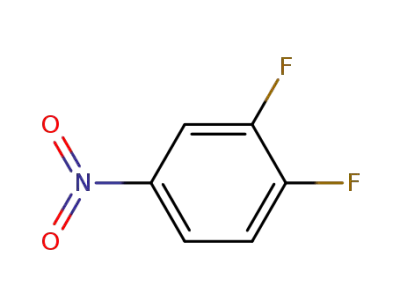
771-69-7
2,3,4-trifluoronitrobenzene

-
-
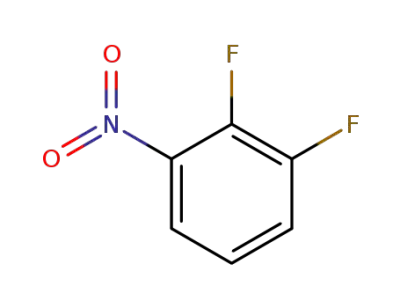
369-34-6
3,4-difluoronitrobenzene

-
-
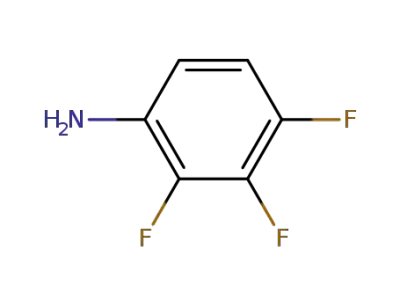
6921-22-8
1,2-difluoro-3-nitrobenzene

-
-
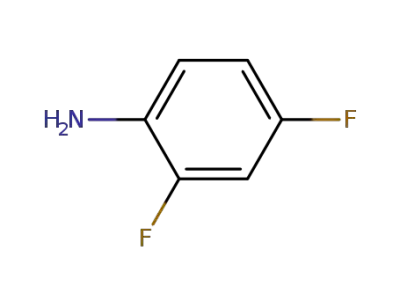
3862-73-5
2,3,4-trifluoroaniline
| Conditions | Yield |
|---|---|
|
With
Dimethylphenylsilane; o-phenylenebis(diphenylphosphine); potassium tert-butylate; copper(l) chloride;
In
tetrahydrofuran;
for 12h;
Overall yield = 95 %Spectr.; regioselective reaction;
Inert atmosphere;
Reflux;
|
-
-
367-25-9,76563-56-9
2,4-difluorophenylamine

-
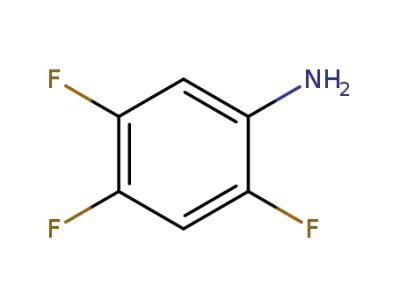
-
367-34-0
2,4,5-trifluoroaniline

-

-
3862-73-5
2,3,4-trifluoroaniline
| Conditions | Yield |
|---|---|
|
With
trifluorormethanesulfonic acid; fluorine;
at 20 ℃;
Title compound not separated from byproducts;
|
3862-73-5 Upstream products
-
771-69-7

2,3,4-trifluoronitrobenzene
-
367-25-9

2,4-difluorophenylamine
-
62-53-3
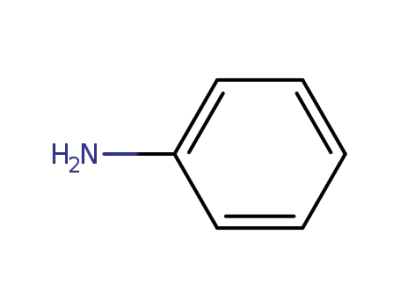
aniline
-
371-40-4
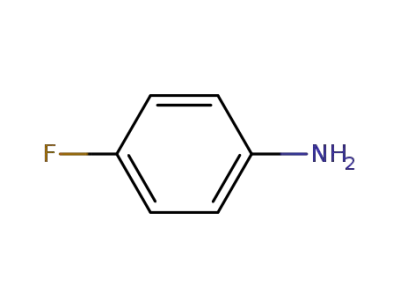
4-fluoroaniline
3862-73-5 Downstream products
-
119474-39-4
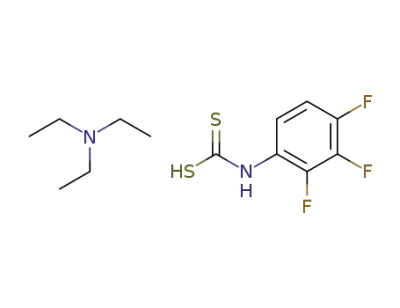
triethylammonium N-(2,3,4-trifluorophenyl)dithiocarbamate
-
140628-52-0
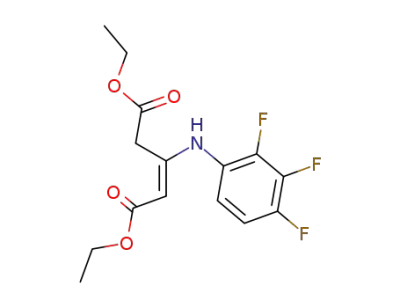
diethyl 3-(2,3,4-trifluoroanilino)-2-pentendioate
-
100501-60-8
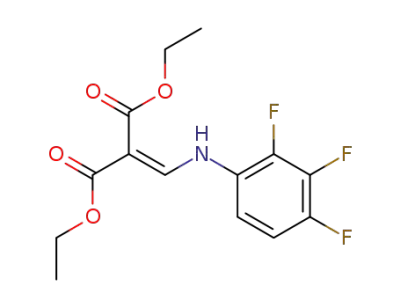
diethyl 2-((2,3,4-trifluorophenylamino)methylene)malonate
-
365-29-7
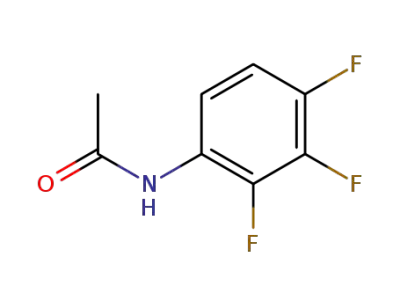
N-(2,3,4-trifluorophenyl)acetamide
Relevant Products
-
1-Butyl-3-methylimidazolium tetrafluoroborate
CAS:174501-65-6
-
3-Bromoaniline
CAS:591-19-5
-
DIBASIC ESTER
CAS:95481-62-2