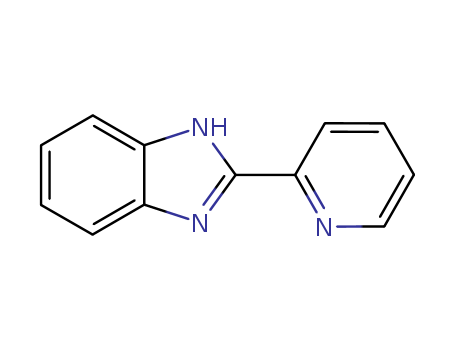
1137-68-4
- Product Name:2-(2-Pyridyl)benzimidazole
- Molecular Formula:C12H9N3
- Purity:99%
- Molecular Weight:195.224
Product Details;
CasNo: 1137-68-4
Molecular Formula: C12H9N3
Appearance: Yellow to brown powder
factory and supplier 1137-68-4 2-(2-Pyridyl)benzimidazole in stock
- Molecular Formula:C12H9N3
- Molecular Weight:195.224
- Appearance/Colour:Yellow to brown powder
- Vapor Pressure:1.51E-07mmHg at 25°C
- Melting Point:218-220 °C(lit.)
- Refractive Index:1.699
- Boiling Point:428.496 °C at 760 mmHg
- PKA:pK1:5.58(+1) (25°C,μ=0.16)
- Flash Point:206.767 °C
- PSA:41.57000
- Density:1.271 g/cm3
- LogP:2.62490
2-(2-PYRIDYL)BENZIMIDAZOLE(Cas 1137-68-4) Usage
InChI:InChI=1/C12H9N3/c1-2-6-10-9(5-1)14-12(15-10)11-7-3-4-8-13-11/h1-8H,(H,14,15)
1137-68-4 Relevant articles
Dehydrogenative synthesis of benzimidazoles under mild conditions with supported iridium catalysts
Tateyama, Keisuke,Wada, Kenji,Miura, Hiroki,Hosokawa, Saburo,Abe, Ryu,Inoue, Masashi
, p. 1677 - 1684 (2016)
Solid supported iridium catalysts, which...
Facile route for the synthesis of benzothiazoles and benzimidazoles in the presence of tungstophosphoric acid impregnated zirconium phosphate under solvent-free conditions
Aliyan, Hamid,Fazaeli, Razieh,Fazaeli, Nahid,Mssah, Ahmad Reza,Naghash, Hamid Javaherian,Alizadeh, Mohammad,Emami, Ginous
, p. 202 - 207 (2009)
Rapid and efficient condensation reactio...
An efficient and one-pot synthesis of imidazolines and benzimidazoles via anaerobic oxidation of carbon-nitrogen bonds in water
Gogoi, Pranjal,Konwar, Dilip
, p. 79 - 82 (2006)
The system, I2/KI/K2CO3/H2O, oxidizes ca...
Preparation of 2-Arylbenzimidazole derivatives using CuO nanoparticles /H2O2 system
Fazlinia, Abbas,Sheikh, Setareh
, p. 126 - 130 (2018)
CuO nanoparticles were prepared in water...
Efficient and high colour-purity green-light polymer light-emitting diodes (PLEDs) based on a PVK-supported Tb3+-containing metallopolymer
Liu, Lin,Pang, Mengyuan,Chen, Hongting,Fu, Guorui,Li, Baoning,Lü, Xingqiang,Wang, Lei
, p. 9021 - 9027 (2017)
Based on the free-radical copolymerizati...
Heteroleptic ruthenium(II) complexes of 2-(2-pyridyl)benzimidazoles: A study of catalytic efficiency towards transfer hydrogenation of acetophenone
Dayan, Osman,Demirmen, Selin,?zdemir, Namik
, p. 926 - 932 (2015)
Six ruthenium(II) complexes ([RuCl2(p-cy...
Semi-empirical computation on mechanism of imidazolines and benzimidazoles synthesis and their QSAR studies
Hazarika, Swapnali,Konwar, Dilip,Bora, Manas Jyoti
, p. 5073 - 5078 (2014)
A green, mild and anaerobic synthesis of...
Reusable α-MoO3 nanobelts catalyzes the green and heterogeneous condensation of 1,2-diamines with carbonyl compounds
Jafarpour, Maasoumeh,Rezaeifard, Abdolreza,Ghahramaninezhad, Mahboube,Tabibi, Tooba
, p. 2087 - 2095 (2013)
Crystalline nanobelts of α-MoO3 have bee...
Mild and highly efficient protocol for the synthesis of benzimidazoles using samarium triflate [Sm(OTf)3]
Narsaiah, A. Venkat,Reddy, A. Ramesh,Yadav
, p. 262 - 267 (2011)
One-pot synthesis of benzimidazole compo...
Acidic properties of benzimidazoles and substituent effects. II. The substituent effect on the imidazole formation from p-substituted o-phenylenediamines and on the acid dissociation of the benzimidazoles
Ichikawa,Hisano
, p. 358 - 361 (1977)
-
Anticancer copper pyridine benzimidazole complexes: ROS generation, biomolecule interactions, and cytotoxicity
Prosser, Kathleen E.,Chang, Stephanie W.,Saraci, Felix,Le, Phuc H.,Walsby, Charles J.
, p. 89 - 99 (2017)
The Cu(II) complex CuCl2(pbzH), pbzH = 2...
Electrocatalytic CO2 reduction using rhenium(I) complexes with modified 2-(2'-pyridyl)imidazole ligands
Sinha, Soumalya,Berdichevsky, Ellan K.,Warren, Jeffrey J.
, p. 63 - 68 (2017)
The reduction of CO2 to CO is an ongoing...
Ti, Zr and v complexes with N-allyl functionalized heterocyclic ligands as catalysts for ethylene polymerization Dedicated to Professor Max Herberhold on the occasion of his 80th birthday (August 02, 2016).
Elagab, Hamdi Ali,Alt, Helmut G.
, p. 17 - 29 (2016)
Titanium, zirconium and vanadium complex...
High-efficiency electrophosphorescent copolymers containing charged iridium complexes in the side chains
Du, Bin,Wang, Lei,Wu, HongBin,Yang, Wei,Zhang, Yong,Liu, RanSheng,Sun, MingLiang,Peng, Junbiao,Cao, Yong
, p. 7432 - 7442 (2007)
A convenient approach to novel charged I...
Iron sulfide catalyzed redox/condensation cascade reaction between 2-amino/hydroxy nitrobenzenes and activated methyl groups: A straightforward atom economical approach to 2-hetaryl-benzimidazoles and -benzoxazoles
Nguyen, Thanh Binh,Ermolenko, Ludmila,Al-Mourabit, Ali
, p. 118 - 121 (2013)
Iron sulfide generated in situ from elem...
-
Walter,Freiser
, p. 217,218 (1954)
-
Two new mixed copper(ii)-dipeptide complexes of N, N -donor heterocycle ligands: Studies on their non-covalent DNA binding, chemical nuclease, antioxidant and anticancer activities
Gan, Qian,Zhang, Chun-Lian,Wang, Bing-Feng,Xiong, Ya-Hong,Fu, Yin-Lian,Mao, Zong-Wan,Le, Xue-Yi
, p. 35952 - 35965 (2016)
Two novel mononuclear mixed ligand coppe...
Mesoporous silica supported ytterbium as catalyst for synthesis of 1,2-disubstituted benzimidazoles and 2-substituted benzimidazoles
Samanta, Partha Kumar,Banerjee, Rumeli,Richards, Ryan M.,Biswas, Papu
, (2018)
The benzimidazole ring is an important p...
A comparative study of the optical and electroluminescent properties of EuIII complexes with TTA and 2-(2′-pyridyl)azoles: The crystal structure of [Eu(TTA)3(PBO)]
Gao, Li-Hua,Guan, Min,Wang, Ke-Zhi,Jin, Lin-Pei,Huang, Chun-Hui
, p. 3731 - 3737 (2006)
Two EuIII mixed-ligand complexes, namely...
Reaction intermediates of quinol oxidation in a photoactivatable system that mimics electron transfer in the cytochrome bc1 complex
Cape, Jonathan L.,Bowman, Michael K.,Kramer, David M.
, p. 4208 - 4215 (2005)
Current competing models for the two-ele...
The synthesis of azo compounds from nitro compounds using lead and triethylammonium formate
Alloum, Abdelkrim Ben,Bougrin, Khalid,Soufiaoui, Mohamed
, p. 5935 - 5937 (2003)
Chemoselective synthesis of benzimidazol...
Single-component Eu3+-Tb3+-Gd3+-grafted polymer with ultra-high color rendering index white-light emission
Liu, Lin,Fu, Guorui,Li, Baoning,Lü, Xingqiang,Wong, Wai-Kwok,Jones, Richard A.
, p. 6762 - 6771 (2017)
Through an approach of pre-coordination ...
Photophysics of Three Pyridylbenzimidazoles in Solution
Brown, Robert G.,Entwistle, Neil,Hepworth, John D.,Hodgson, Kevin W.,May, Bernadette
, p. 2418 - 2420 (1982)
Fluorescence decay time data are present...
Synthesis and electron-transfer properties of benzimidazole-functionalized ruthenium complexes for highly efficient dye-sensitized solar cells
Huang, Wei-Kai,Cheng, Chi-Wen,Chang, Shu-Mei,Lee, Yuan-Pern,Diau, Eric Wei-Guang
, p. 8992 - 8994 (2010)
Novel heteroleptic ruthenium complexes -...
Imidazolium chloride-catalyzed synthesis of benzimidazoles and 2-substituted benzimidazoles from o-phenylenediamines and DMF derivatives
Gan, Zongjie,Tian, Qingqiang,Shang, Suqin,Luo, Wen,Dai, Zeshu,Wang, Huajun,Li, Dan,Wang, Xuetong,Yuan, Jianyong
, p. 7450 - 7456 (2018)
A facile, general, and economical synthe...
Cu(I) complexes regulated by N-heterocyclic ligands: Syntheses, structures, fluorescence and electrochemical properties
Mao, Shanshan,Han, Xintong,Li, Chuang,Xu, Yuling,Shen, Kesheng,Shi, Xinkui,Wu, Huilu
, p. 408 - 414 (2018)
Three mononuclear Cu(I) complexes, namel...
Amide-imine conjugate involving gallic acid and naphthalene for nano-molar detection, enrichment and cancer cell imaging of La3+: Studies on the catalytic activity of the La3+complex
Shaikh, Ahad,Ghosh, Milan,Mukherjee, Pallabi,Ghosh, Avijit,Molla, Rostam Ali,Ta, Sabyasachi,Das, Debasis
, p. 13501 - 13506 (2020)
A single crystal X-ray structurally char...
Microwave-assisted synthesis of 2-(2-pyridyl)azoles. Study of their corrosion inhibiting properties
Likhanova, Natalya V.,Veloz, M. Aurora,Hoepfl, Herbert,Matias, Diana J.,Reyes-Cruz, Victor E.,Olivares, Octavio,Martinez-Palou, Rafael
, p. 145 - 153 (2007)
(Chemical Equation Presented) An efficie...
Synthesis and antiinflammatory activity of some 2-(substituted-pyridinyl)benzimidazoles
Tsukamoto,Yoshino,Kohno,Ohtaka,Kagaya,Ito
, p. 734 - 738 (1980)
A series of 2-(2-pyridinyl)benzimidazole...
Antimicrobial evaluation of myricetin derivatives containing benzimidazole skeleton against plant pathogens
Chen, Mei,Tang, Xuemei,Liu, Tingting,Peng, Feng,Zhou, Qing,Luo, Hui,He, Ming,Xue, Wei
, (2021)
A series of novel myricetin derivatives ...
Electrocatalytic CO2 Reduction with a Half-Sandwich Cobalt Catalyst: Selectivity towards CO
Kumar Pandey, Indresh,Kumar, Abhishek,Choudhury, Joyanta
, p. 904 - 909 (2020)
We present herein a Cp*Co(III)-half-sand...
Copper-catalyzed one-pot synthesis of benzimidazole derivatives
Sharghi, Hashem,Hosseini-Sarvari, Mona,Moeini, Fatemeh
, p. 1044 - 1051 (2008)
A simple, efficient, and environmentally...
Novel dye sensitizers of main chain polymeric metal complexes based on complexes of 2-(2′-pyridyl)benzimidazole derivative with Zn(II), Co(II): Synthesis, characterization, and photovoltaic performance for dye-sensitized solar cells
Peng, Dahai,Zhang, Wei,Tang, Guipeng,Zhou, Jun,Hu, Jiaomei,Xie, Qiufang,Zhong, Chaofan
, p. 397 - 404 (2015)
In this paper, four novel D-π-A polymeri...
Silphox [POCl3-n(SiO2)n] as a new, efficient, and heterogeneous reagent for the synthesis of benzimidazole derivatives under microwave irradiation
Hasaninejad, Alireza,Niknam, Khodabakhsh,Zare, Abdolkarim,Farsimadan, Ehsan,Shekouhy, Mohsen
, p. 147 - 155 (2009)
Silphox [POCl3-n(SiO2)n] efficiently cat...
Concentrated solar radiation promoted unconventional greener approach: Solvent-free benign synthesis of functionalized benzimidazoles
Harsh, Simran,Yusuf, Mohamad,Sharma, Rohit,Kumar, Yogesh,Kumar, Rupesh
, p. 119 - 130 (2018)
Renewable concentrated solar-radiation (...
Titanium, zirconium and vanadium complexes of 2-(benzimidazolyl, benzothiazolyl, and benzoxazolyl) pyridine as catalyst precursors for ethylene polymerization
Elagab, Hamdi Ali,Alt, Helmut G.
, p. 266 - 275 (2015)
Dissymmetric chelating complexes of Ti, ...
Structure-activity relationships in cytotoxic AuI/Au III complexes derived from 2-(2′-pyridyl)benzimidazole
Maiore, Laura,Aragoni, Maria Carla,Deiana, Carlo,Cinellu, Maria Agostina,Isaia, Francesco,Lippolis, Vito,Pintus, Anna,Serratrice, Maria,Arca, Massimiliano
, p. 4068 - 4080 (2014)
Gold(I) and gold(III) complexes derived ...
Photoluminescence and electrochemical studies of tetranuclear ruthenium(II) polypyridyl complexes of benzimidazolyl functionalised pyrenylcalix[4]resorcinarene
Louis, Ligimol,Alexander,Kumar, D. Suresh,Senthan, S. Amudhan,Viveke, A. Arun
, p. 245 - 251 (2019)
We report the hitherto unreported pyrene...
[Diaquo{bis(p-hydroxybenzoato-κ1O1)}(1-methylimidazole- κ1N1)}copper(II)]: Synthesis, crystal structure, catalytic activity and DFT study
Brahman, Dhiraj,Chhetri, Sailesh,Kamath, Amarjit,McArdle, Patrick,Sinha, Biswajit
, (2021/09/04)
Metal-organic hybrid complexes often exh...
A heterogeneous catalytic strategy for facile production of benzimidazoles and quinoxalines from primary amines using the Al-MCM-41 catalyst
Vasu, Amrutham,Naresh, Mameda,Krishna Sai, Gajula,Divya Rohini, Yennamaneni,Murali, Boosa,Ramulamma, Madasu,Ramunaidu, Addipilli,Narender, Nama
, p. 9439 - 9446 (2021/12/09)
This study reports a straightforward het...
Preparation method of 2-substituted benzimidazole compound
-
Paragraph 0084-0088, (2021/02/20)
The invention discloses a preparation me...
s-Tetrazine-functionalized hyper-crosslinked polymers for efficient photocatalytic synthesis of benzimidazoles
An, Wan-Kai,Zheng, Shi-Jia,Zhang, Hui-Xing,Shang, Tian-Tian,Wang, He-Rui,Xu, Xiao-Jing,Jin, Qiu,Qin, Yuchen,Ren, Yunlai,Jiang, Song,Xu, Cui-Lian,Hou, Mao-Song,Pan, Zhenliang
supporting information, p. 1292 - 1299 (2021/02/26)
Developing green-safe, efficient and rec...
1137-68-4 Process route
-
-
3731-51-9
2-(Aminomethyl)pyridine

-
-
95-54-5
1,2-diamino-benzene

-
-
1137-68-4
2-(2'-pyridyl)benzimidazole
| Conditions | Yield |
|---|---|
|
With
Al cations incorporated into the Si framework; open air;
In
neat (no solvent);
at 100 ℃;
for 15h;
Green chemistry;
|
93% |
|
With
iron(III) chloride; 3-methyl-4-oxa-5-azahomoadamantane; oxygen;
In
water;
at 100 ℃;
for 16h;
|
86% |
|
With
sulfur;
In
neat (no solvent);
at 110 ℃;
for 16h;
Inert atmosphere;
|
85% |
|
With
2,2,6,6-tetramethyl-piperidine-N-oxyl; ferric nitrate;
In
neat (no solvent);
at 110 ℃;
for 28h;
Green chemistry;
|
85% |
|
With
copper(ll) bromide;
In
toluene;
at 100 ℃;
for 24h;
|
82% |
|
With
iron(III) sulfate; 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical;
In
neat (no solvent);
at 110 ℃;
for 30h;
Green chemistry;
|
69% |
|
With
tert.-butylhydroperoxide;
In
water;
at 100 ℃;
for 10h;
|
68% |
-
-
98-98-6
2-Picolinic acid

-

-
95-54-5
1,2-diamino-benzene

-

-
1137-68-4
2-(2'-pyridyl)benzimidazole
| Conditions | Yield |
|---|---|
|
With
polyphposphoric acid;
at 180 ℃;
for 2h;
|
93% |
|
With
bis(acetylacetonate)oxidovanadium(IV);
for 0.05h;
Microwave irradiation;
Neat (no solvent);
|
92% |
|
With
silphox [POCl3-n(SiO2)n, silica phosphinoxide];
In
N,N-dimethyl-formamide;
for 0.133333h;
Microwave irradiation;
|
89% |
|
With
phosphoric acid;
at 250 ℃;
for 2h;
|
85% |
|
In
various solvent(s);
at 180 - 200 ℃;
for 8h;
|
85% |
|
With
polyphosphoric acid;
at 180 ℃;
for 8h;
Inert atmosphere;
|
85% |
|
With
polyphosphoric acid;
at 175 ℃;
Inert atmosphere;
|
85% |
|
With
polyphosphoric acid;
at 180 ℃;
for 6h;
Inert atmosphere;
|
85% |
|
With
PPA;
at 160 ℃;
for 4h;
|
74% |
|
With
PPA;
at 150 ℃;
for 2h;
Inert atmosphere;
|
62% |
|
2-Picolinic acid; 1,2-diamino-benzene;
at 200 ℃;
for 4h;
Inert atmosphere;
With
sodium hydroxide;
In
water;
Cooling with ice;
Inert atmosphere;
|
58% |
|
With
polyphosphoric acid;
at 150 ℃;
for 4h;
|
56.7% |
|
With
phosphoric acid;
at 180 ℃;
for 24h;
|
52% |
|
With
polyphosphoric acid;
at 160 ℃;
for 8h;
|
43% |
|
With
polyphosphoric acid;
In
ethylene glycol;
at 80 - 200 ℃;
|
36.2% |
|
With
PPA;
at 190 ℃;
for 8h;
|
25% |
|
With
PPA;
at 100 ℃;
|
21% |
|
at 310 ℃;
|
|
|
at 220 ℃;
|
|
|
With
PPA;
|
|
|
|
|
|
With
phosphoric acid;
at 160 ℃;
|
|
|
2-Picolinic acid; 1,2-diamino-benzene;
at 170 ℃;
for 4h;
With
sodium hydrogencarbonate;
In
water;
pH=7;
|
|
|
With
polyphosphoric acid;
at 150 ℃;
for 8h;
Inert atmosphere;
|
|
|
With
polyphosphoric acid;
at 180 ℃;
|
|
|
With
polyphosphoric acid;
for 3h;
Reflux;
|
|
|
With
polyphosphoric acid;
at 220 ℃;
for 6h;
|
|
|
With
polyphosphoric acid;
at 180 ℃;
|
|
|
With
polyphosphoric acid PPA;
at 180 ℃;
Inert atmosphere;
|
|
|
With
polyphosphoric acid;
at 180 ℃;
|
|
|
With
polyphosphoric acid;
at 175 ℃;
|
|
|
With
phosphoric acid;
In
N,N-dimethyl-formamide;
|
|
|
2-Picolinic acid; 1,2-diamino-benzene;
With
sulfolane; methanesulfonic acid;
In
water;
at 110 ℃;
for 8h;
With
acetic acid;
In
water;
for 3h;
Reflux;
|
1137-68-4 Upstream products
-
109-06-8
α-picoline
-
95-54-5

1,2-diamino-benzene
-
1121-60-4
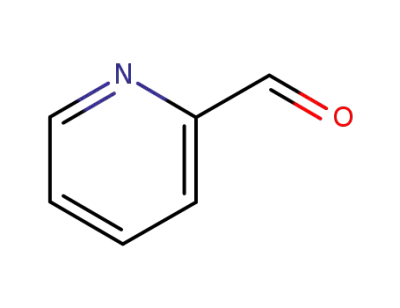
pyridine-2-carbaldehyde
-
98-98-6

2-Picolinic acid
1137-68-4 Downstream products
-
74356-96-0
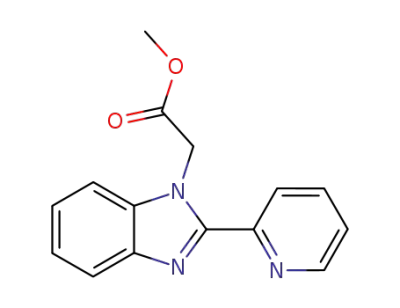
(2-[2]pyridyl-benzimidazol-1-yl)-acetic acid methyl ester
-
34707-81-8
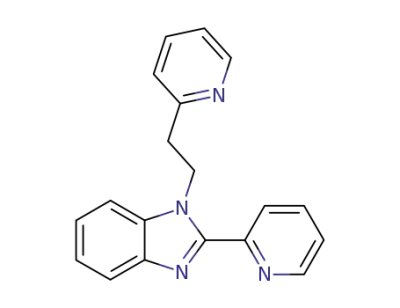
1-<2-(2-Pyridyl)ethyl>-2-(2-pyridyl)benzimidazol
-
34707-82-9
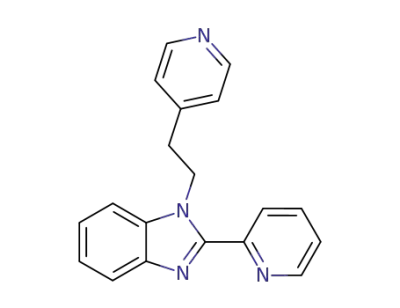
2-pyridin-2-yl-1-(2-pyridin-4-yl-ethyl)-1H-benzoimidazole
-
51759-60-5

5-nitro-2-(pyridin-2-yl)-1H-benzo[d]imidazole
Relevant Products
-
2,5-Dimethoxy-Beta-Nitrostyrene
CAS:40276-11-7
-
5-Bromo-2-chlorobenzotrifluoride
CAS:445-01-2
-
Tris(4-methoxyphenyl)phosphine
CAS:855-38-9