350-30-1
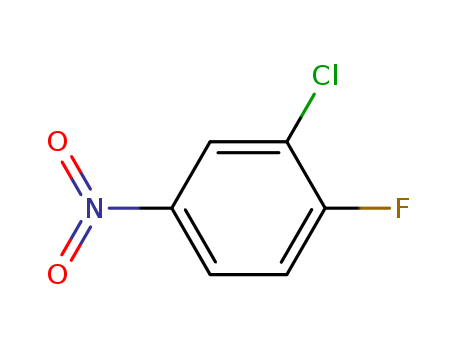
- Product Name:3-Chloro-4-fluoronitrobenzene
- Molecular Formula:C6H3ClFNO2
- Purity:99%
- Molecular Weight:175.547
Product Details;
CasNo: 350-30-1
Molecular Formula: C6H3ClFNO2
Appearance: white to light yellow crystal powder
factory and supplier 350-30-1 3-Chloro-4-fluoronitrobenzene in stock
- Molecular Formula:C6H3ClFNO2
- Molecular Weight:175.547
- Appearance/Colour:white to light yellow crystal powder
- Vapor Pressure:0.0893mmHg at 25°C
- Melting Point:40-42 °C(lit.)
- Refractive Index:1.554
- Boiling Point:232.5 °C at 760 mmHg
- Flash Point:94.4 °C
- PSA:45.82000
- Density:1.494 g/cm3
- LogP:2.91050
3-Chloro-4-fluoronitrobenzene(Cas 350-30-1) Usage
|
Synthesis |
In certain instances, when both chloro and nitro may act as a leaving group, a surprising selectivity of one over the other is observed, for example chloro-2,5- dinitrobenzene was converted to 4-fluoro-3-chloronitrobenzene. |
|
Synthesis Reference(s) |
The Journal of Organic Chemistry, 63, p. 8448, 1998 DOI: 10.1021/jo981557oSynthetic Communications, 21, p. 505, 1991 DOI: 10.1080/00397919108016776 |
|
General Description |
2-Chloro-1-fluoro-4-nitrobenzene undergoes reduction in the presence of Mo(CO)6 and 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU) under microwave irradiation to yield a mixture of 3-chloro-4-fluoroaniline and 3-chloro-4-ethoxyaniline. |
InChI:InChI=1/C6H3ClFNO2/c7-5-3-4(9(10)11)1-2-6(5)8/h1-3H
350-30-1 Relevant articles
Catalyst and application thereof in synthesis of aromatic fluorine compounds
-
Paragraph 0042-0044, (2020/11/10)
The invention belongs to the field of ca...
Preparation method of 3-chloro-4-fluoronitrobenzene
-
Paragraph 0012; 0013; 0014; 0015; 0016; 0017; 0018, (2017/08/29)
The invention relates to a preparation m...
O-dichlorobenzene with a quinolone drugs for coproduction method of key intermediate
-
Paragraph 0020; 0066-0067, (2017/01/19)
The invention relates to the field of me...
Phosphonium ionic liquids as highly thermal stable and efficient phase transfer catalysts for solid-liquid Halex reactions
Fan, Ao,Chuah, Gaik-Khuan,Jaenicke, Stephan
, p. 300 - 304 (2013/01/15)
Trihexyl (tetradecyl) phosphonium tetraf...
350-30-1 Process route
-
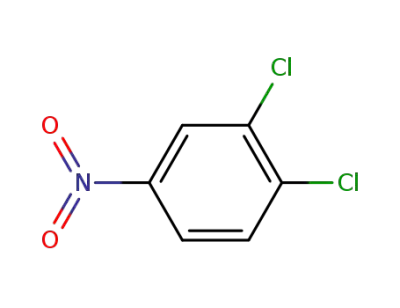
-
99-54-7
3,4-dichloronitrobenzene

-
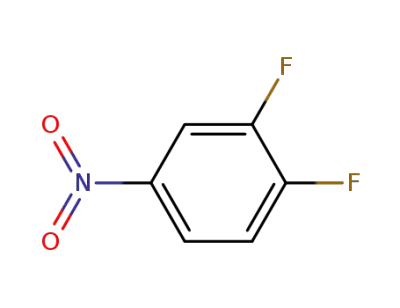
-
369-34-6
3,4-difluoronitrobenzene

-
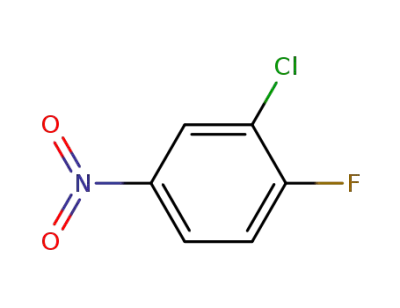
-
350-30-1
3-chloro-4-fluoronitrobenzene
| Conditions | Yield |
|---|---|
|
With
potassium fluoride; tetraphenylphosphonium bromide;
at 175 - 195 ℃;
for 30h;
Yields of byproduct given;
|
79% |
|
With
potassium fluoride; 2-(4-chlorophenyl)propene copolymer-grafted tetraphenylphosphonium chloride; cross-linked styrene;
In
sulfolane;
at 210 ℃;
for 7h;
|
64% 12% |
|
With
potassium fluoride; tetraphenylphosphonium bromide;
at 175 - 195 ℃;
for 30h;
Yield given. Yields of byproduct given;
|
-

-
99-54-7
3,4-dichloronitrobenzene

-

-
350-30-1
3-chloro-4-fluoronitrobenzene
| Conditions | Yield |
|---|---|
|
With
trihexyl (tetradecyl) phosphonium tetrafluoroborate; potassium fluoride;
In
dimethyl sulfoxide;
at 180 ℃;
Solvent;
Reagent/catalyst;
Time;
|
100% |
|
With
potassium fluoride; water; tetramethlyammonium chloride;
In
dimethyl sulfoxide;
at 120 ℃;
for 3.5h;
Rate constant;
various solvents; other phase transfer catalyst; also without phase transfer catalyst;
|
99% |
|
With
potassium fluoride; water; tetramethlyammonium chloride;
In
acetic acid;
at 120 ℃;
for 3.5h;
|
99% |
|
With
potassium fluoride; bis(tricyclohexylphosphine)nickel(II) dichloride; tetrabutyl ammonium fluoride;
In
N,N-dimethyl-formamide;
at 150 ℃;
for 8h;
Inert atmosphere;
|
99.1% |
|
With
potassium fluoride; polydiallyldimethylammonium chloride;
In
dimethyl sulfoxide;
for 0.166667h;
Heating;
microwave irradiation;
|
94.5% |
|
With
potassium fluoride; bis(triphenylphosphine)iminium chloride;
In
dimethyl sulfoxide;
at 150 ℃;
for 8h;
Inert atmosphere;
|
91% |
|
3,4-dichloronitrobenzene;
With
potassium fluoride;
at 140 - 150 ℃;
for 3h;
With
tetramethlyammonium chloride;
at 165 - 170 ℃;
for 7h;
Temperature;
|
87% |
|
With
potassium fluoride; 2-(4-chlorophenyl)propene copolymer-grafted tetraphenylphosphonium chloride; cross-linked styrene;
In
sulfolane;
at 160 ℃;
for 3h;
|
85% |
|
With
potassium fluoride; PEG 5090;
In
dimethyl sulfoxide;
at 153 ℃;
for 13h;
|
84% |
|
With
potassium fluoride;
In
dichloromethane; water;
|
70% |
|
With
potassium fluoride;
In
sulfolane;
|
63.5% |
|
With
potassium fluoride;
|
48.1% |
|
With
potassium fluoride;
copolymer of 1,4-(imidazol-1-yl)2(CH2)4 and 1,2-Br2-ethane;
In
dimethyl sulfoxide;
at 206 ℃;
for 0.5h;
microwave irradiation;
|
87.4 % Chromat. |
|
With
KF;
In
sulfolane;
|
|
|
With
potassium fluoride;
|
|
|
With
potassium fluoride; cesium fluoride;
In
trimethylbenzylammonium bromide;
|
|
|
With
potassium fluoride;
In
dimethyl sulfoxide; benzene;
|
|
|
With
KF;
In
thiophene; Polytetrafluoroethylene;
|
|
|
With
potassium fluoride;
In
sulfolane;
|
|
|
With
potassium fluoride; cesium fluoride;
In
sulfolane;
|
|
|
With
potassium fluoride; cesium fluoride;
In
N-methyl-acetamide;
|
|
|
With
potassium fluoride;
tetrabutylammomium bromide;
In
dimethyl sulfoxide; toluene;
at 120 - 200 ℃;
Product distribution / selectivity;
|
|
|
With
potassium fluoride; C58H82N4O8;
In
dimethyl sulfoxide;
at 40 ℃;
for 24h;
|
98.8 %Chromat. |
350-30-1 Upstream products
-
348-51-6
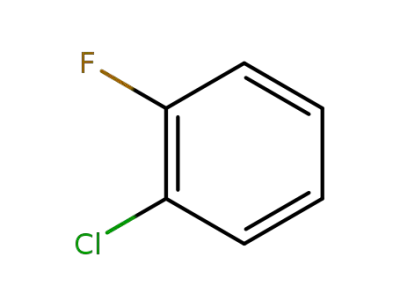
1-chloro-2-fluorobenzene
-
350-46-9
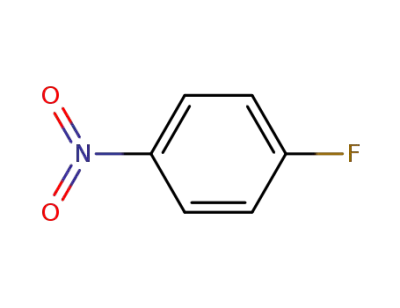
4-Fluoronitrobenzene
-
99-54-7

3,4-dichloronitrobenzene
-
382-76-3
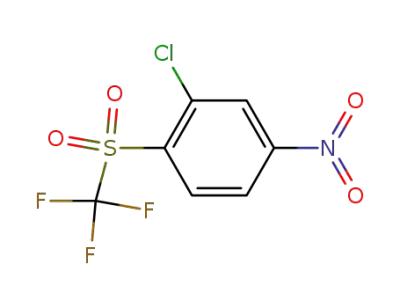
2-chloro-4-nitro-1-(trifluoromethylsulfonyl)benzene
350-30-1 Downstream products
-
367-21-5
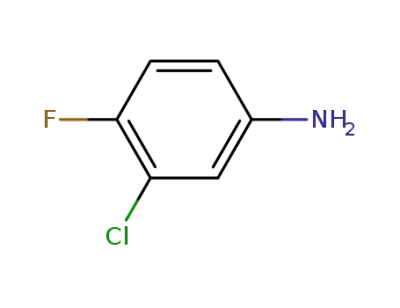
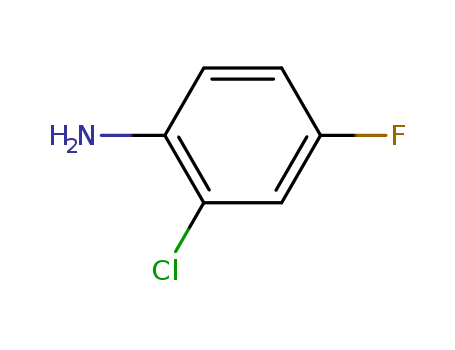
3-chloro-4-fluorophenylamine
-
65976-57-0
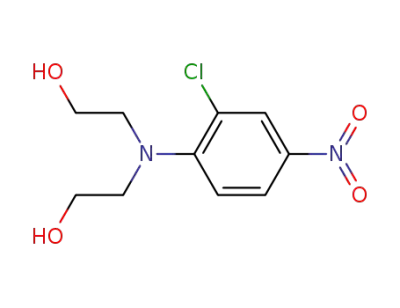
2-[(2-Chloro-4-nitro-phenyl)-(2-hydroxy-ethyl)-amino]-ethanol
-
29482-57-3
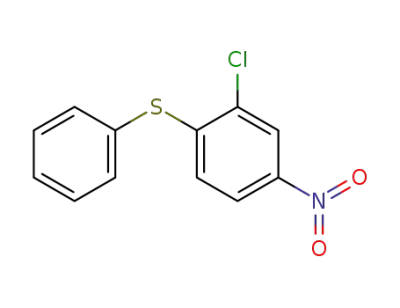
2-chloro-4-nitro-1-phenylsulfanylbenzene
-
77146-55-5
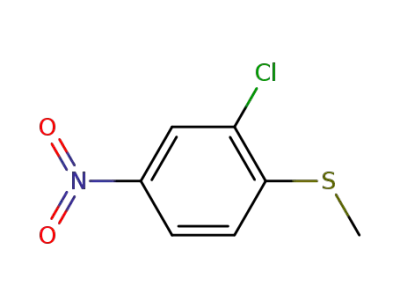
2-chloro-1-(methylsulfanyl)-4-nitrobenzene
Relevant Products
-
2-Chloro-4-fluoroaniline
CAS:2106-02-7
-
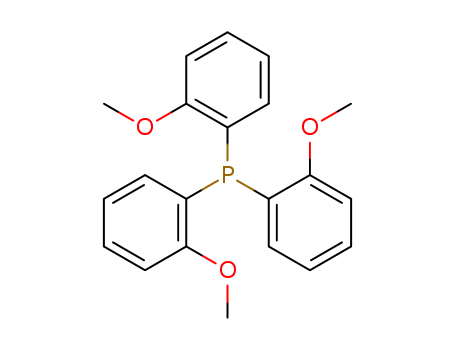
Tris(2-methoxyphenyl)phosphine
CAS:4731-65-1
-
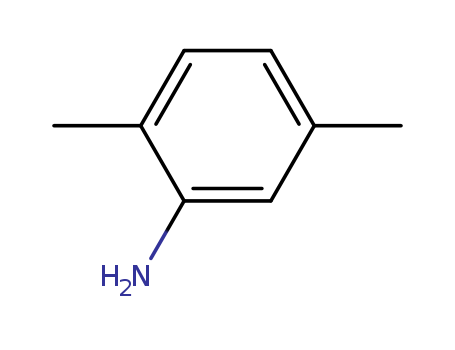
2,5-Dimethylaniline
CAS:95-78-3