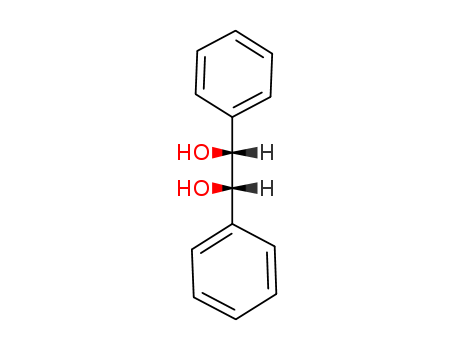
579-43-1
- Product Name:Meso-hydrobenzoin
- Molecular Formula:C14H14O2
- Purity:99%
- Molecular Weight:214.264
Product Details;
CasNo: 579-43-1
Molecular Formula: C14H14O2
Appearance: White crystalline powder
factory and supplier 579-43-1 Meso-hydrobenzoin in stock
- Molecular Formula:C14H14O2
- Molecular Weight:214.264
- Appearance/Colour:White crystalline powder
- Vapor Pressure:3.18E-06mmHg at 25°C
- Melting Point:137-139 °C(lit.)
- Refractive Index:1.624
- Boiling Point:373 °C at 760 mmHg
- PKA:13.38±0.20(Predicted)
- Flash Point:179.8 °C
- PSA:40.46000
- Density:1.193 g/cm3
- LogP:2.45360
meso-1,2-Diphenyl-1,2-ethanediol(Cas 579-43-1) Usage
|
Purification Methods |
meso-Hydrobenzoin [579-43-1] M 214.3, m 139o, 139-140o. Crystallise it from EtOH or water. [Beilstein 6 H 1003, 6 I 490, 6 II 967. 6 III 5429, 6 IV 6682.] |
|
General Description |
Desymmetrization of meso-hydrobenzoin using chiral phosphine catalyst has been reported. Conversion of meso-hydrobenzoin to trans-stillbene oxide by treatment with an aryl sulfonyl chloride and aqueous sodium hydroxide has been reported. |
InChI:InChI=1/C14H14O2/c15-13(11-7-3-1-4-8-11)14(16)12-9-5-2-6-10-12/h1-10,13-16H/t13-,14+
579-43-1 Relevant articles
Diastereoselective Ce(III)-catalyzed pinacol couplings of aldehydes
Groth,Jeske
, p. 129 - 131 (2001)
Aliphatic and aromatic aldehydes were co...
Asymmetric hydrogenation of 1,4-diketones: facile synthesis of enantiopure 1,4-diarylbutane-1,4-diols
Huang, Fanping,Shao, Pan-Lin,Song, Jingyuan,Wang, Jiang,Zhang, Xumu
supporting information, p. 262 - 265 (2022/01/06)
Owing to the biological significance and...
Efficient splitting of alcohols into hydrogen and C–C coupled products over ultrathin Ni-doped ZnIn2S4 nanosheet photocatalyst
Li, Jing-Yu,Qi, Ming-Yu,Xu, Yi-Jun
, p. 1084 - 1091 (2022/03/15)
Integrating selective organic synthesis ...
Electrochemical synthesis of quinazolinone: via I2-catalyzed tandem oxidation in aqueous solution
Hou, Huiqing,Ma, Xinhua,Lin, Yingying,Lin, Jin,Sun, Weiming,Wang, Lei,Xu, Xiuzhi,Ke, Fang
, p. 17721 - 17726 (2021/05/29)
The development of protocols for synthes...
Electrochemical Arylation of Aldehydes, Ketones, and Alcohols: from Cathodic Reduction to Convergent Paired Electrolysis
Zhang, Sheng,Li, Lijun,Li, Jingjing,Shi, Jianxue,Xu, Kun,Gao, Wenchao,Zong, Luyi,Li, Guigen,Findlater, Michael
, p. 7275 - 7282 (2021/03/01)
Arylation of carbonyls, one of the most ...
579-43-1 Process route
-
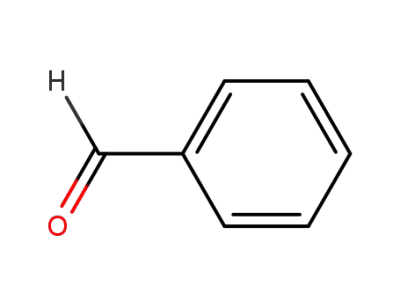
-
100-52-7
benzaldehyde

-
-
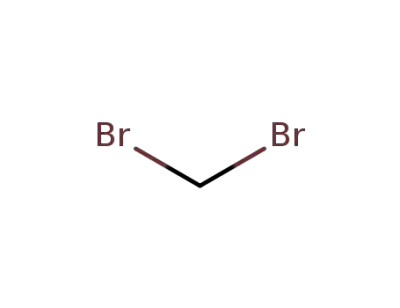
74-95-3
1,1-dibromomethane

-
-
98-85-1,13323-81-4
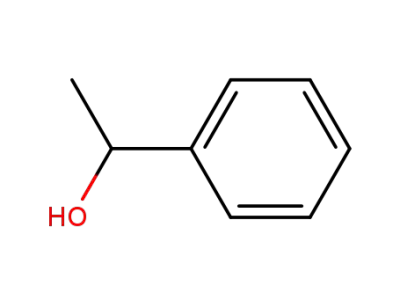
1-Phenylethanol

-
-
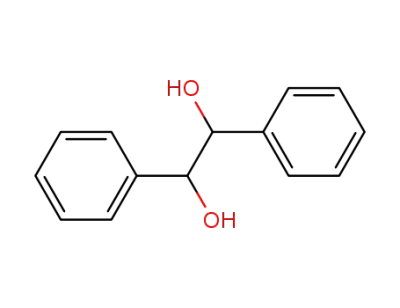
492-70-6,655-48-1,2325-10-2,38270-73-4,52340-78-0,579-43-1
1,2-diphenyl-1,2-ethanediol

-

-
100-51-6,185532-71-2
benzyl alcohol
| Conditions | Yield |
|---|---|
|
With
lithium;
In
tetrahydrofuran;
Ambient temperature;
|
-
-
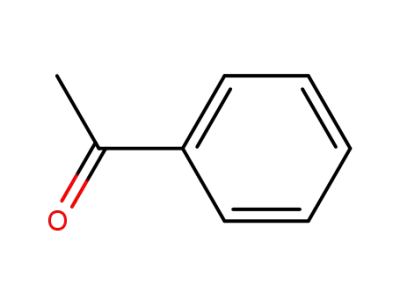
98-86-2
acetophenone

-

-
98-85-1,13323-81-4
1-Phenylethanol

-

-
492-70-6,655-48-1,2325-10-2,38270-73-4,52340-78-0,579-43-1
1,2-diphenyl-1,2-ethanediol
| Conditions | Yield |
|---|---|
|
With
samarium; iodine; isopropyl alcohol;
at 25 ℃;
for 20h;
Further Variations:;
Reagents;
Product distribution;
|
96 % Chromat. 2 % Chromat. |
|
With
Piperonyl butoxide; tert-butylammonium hexafluorophosphate(V);
In
tetrahydrofuran;
at 20 ℃;
Electrochemical reaction;
|
579-43-1 Upstream products
-
79-21-0
peracetic acid
-
645-49-8
cis-stilben
-
103-30-0
(E)-1,2-diphenyl-ethene
-
530-36-9

2-amino-1,2-diphenylethanol
579-43-1 Downstream products
-
451-40-1
phenyl benzyl ketone
-
947-91-1

Diphenylacetaldehyde
-
103-30-0

(E)-1,2-diphenyl-ethene
-
15951-99-2
1,2-dichloro-1,2-diphenylethane
Relevant Products
-
DIBASIC ESTER
CAS:95481-62-2
-
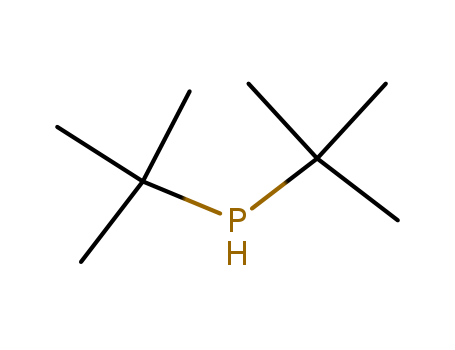
Di-tert-butylphosphine
CAS:819-19-2
-
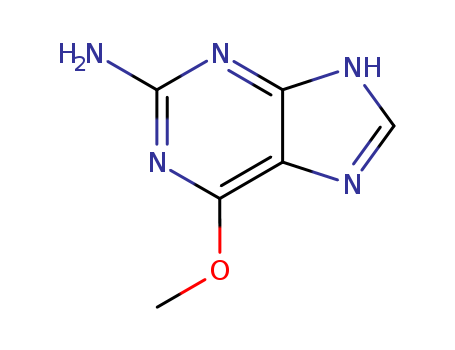
2-Amino-6-methoxypurine
CAS:20535-83-5