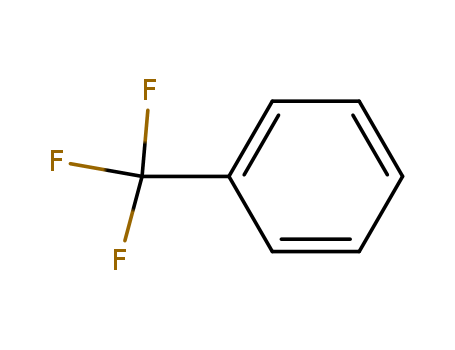
20462-00-4
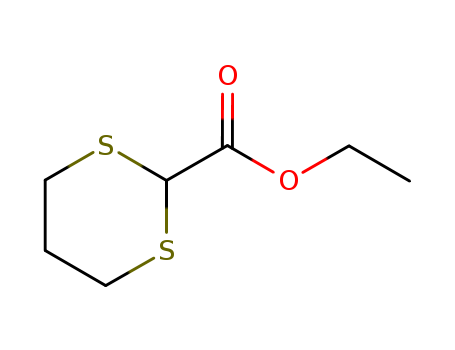
- Product Name:Ethyl 1,3-dithiane-2-carboxylate
- Molecular Formula:C7H12 O2 S2
- Purity:99%
- Molecular Weight:192.303
Product Details;
CasNo: 20462-00-4
Molecular Formula: C7H12 O2 S2
Appearance: Clear light yellow liquid.
factory and supplier 20462-00-4 Ethyl 1,3-dithiane-2-carboxylate in stock
- Molecular Formula:C7H12 O2 S2
- Molecular Weight:192.303
- Appearance/Colour:Clear light yellow liquid.
- Vapor Pressure:0.0035mmHg at 25°C
- Melting Point:19-21°C
- Refractive Index:n20/D 1.539(lit.)
- Boiling Point:75-77 ºC (0.2 mmHg)
- Flash Point:130.2°C
- PSA:76.90000
- Density:1.22
- LogP:1.74570
Ethyl 1,3-dithiane-2-carboxylate(Cas 20462-00-4) Usage
|
Synthesis Reference(s) |
Synthetic Communications, 11, p. 343, 1981 DOI: 10.1080/00397918108063615 |
|
Purification Methods |
Dissolve the ester in CHCl3, wash with aqueous K2CO3, twice with H2O, dry over MgSO4, filter, evaporate and distil the residue. [Eliel & Hartman J Org Chem 37 505 1972, Seebach Synthesis 1 17 1969, Beilstein 19/7 V 227.] |
|
General Description |
Ethyl 1,3-dithiane-2-carboxylate is an α-keto acid equivalent and bulky equivalent of acetate. It participates in syn-selective aldol reactions. It can be prepared from the reaction of ethyl diethoxyacetate and 1,3-propanedithiol in the presence of BF3/Et2O. Asymmetric oxidation of ethyl 1,3-dithiane-2-carboxylate by Modena protocol has been reported to afford trans bis-sulfoxide in 60% yield. Carbanion from ethyl 1,3-dithiane-2-carboxylate may be employed for the preparation of α-keto esters. |
InChI:InChI=1/C7H12O2S2/c1-2-9-6(8)7-10-4-3-5-11-7/h7H,2-5H2,1H3
20462-00-4 Relevant articles
A new synthesis of 2-substituted-1,3-dithianes from trichloromethyl compounds
Rivera, Nancy González,Becerril, David Corona,Guadarrama-Pérez, Carlos,Covarrubias-Zu?iga, Adrian,Avila-Zárraga, José Gustavo,Romero-Ortega, Moisés
, p. 1201 - 1204 (2007)
Trichloromethyl compounds are efficientl...
INHIBITORS OF CBL-B AND METHODS OF USE THEREOF
-
Paragraph 2010, (2019/08/12)
Compounds, compositions, and methods for...
PYRIDAZINONE COMPOUNDS
-
Page/Page column 86-87, (2008/12/07)
The invention is directed to pyridazinon...
PREPARATION OF IMIDAZOLE DERIVATIVES AND METHODS OF USE
-
Page/Page column 48, (2010/02/14)
This invention relates to imidazole comp...
20462-00-4 Process route
-
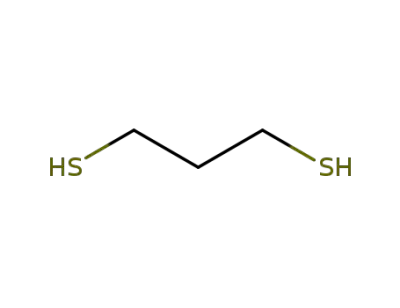
-
109-80-8
1.3-propanedithiol

-
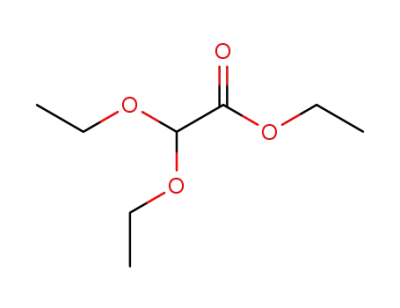
-
6065-82-3
Ethyl diethoxyacetate

-
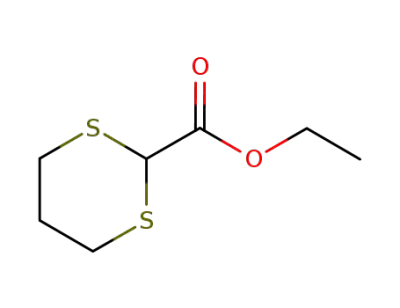
-
20462-00-4
ethyl 1,3-dithiane-2-carboxylate
| Conditions | Yield |
|---|---|
|
With
boron trifluoride diethyl etherate;
In
chloroform;
for 1.5h;
Heating / reflux;
|
94% |
|
With
boron trifluoride diethyl etherate;
In
dichloromethane;
for 14h;
Ambient temperature;
|
60% |
|
1.3-propanedithiol;
With
boron trifluoride diethyl etherate;
In
chloroform;
at -70 ℃;
Ethyl diethoxyacetate;
In
chloroform;
at 70 ℃;
for 1h;
|
47% |
|
With
boron trifluoride diethyl etherate;
In
chloroform;
for 0.5h;
Heating / reflux;
|
37% |
|
|
|
|
With
boron trifluoride diethyl etherate;
In
chloroform;
|
-

-
109-80-8
1.3-propanedithiol

-
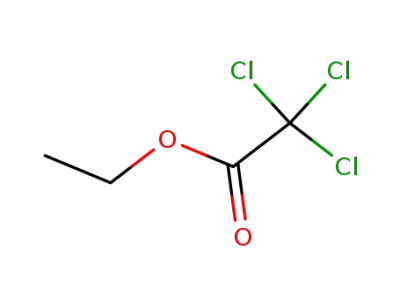
-
515-84-4
Ethyl trichloroacetate

-

-
20462-00-4
ethyl 1,3-dithiane-2-carboxylate
| Conditions | Yield |
|---|---|
|
With
sodium hydride;
In
N,N-dimethyl-formamide;
at 0 ℃;
for 0.25h;
|
85% |
20462-00-4 Upstream products
-
109-80-8

1.3-propanedithiol
-
64805-08-9
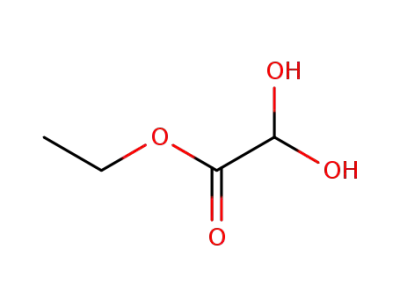
ethyl glyoxylate hydrate
-
535-15-9
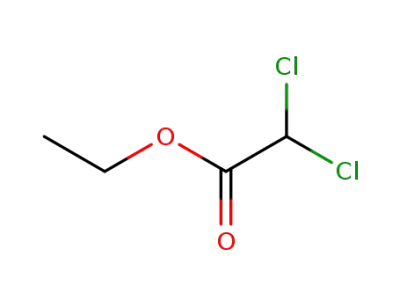
ethyl 1,1-dichloroacetate
-
6065-82-3

Ethyl diethoxyacetate
20462-00-4 Downstream products
-
58810-96-1
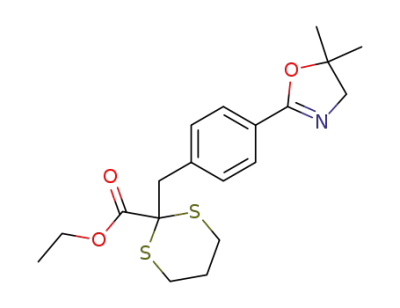
2-[4-(5,5-dimethyl-4,5-dihydro-oxazol-2-yl)-benzyl]-[1,3]dithiane-2-carboxylic acid ethyl ester
-
32557-27-0
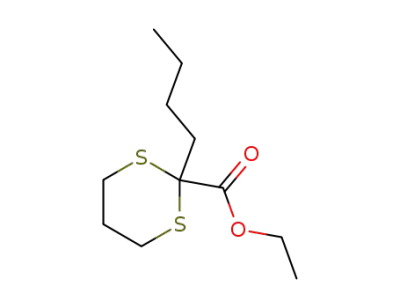
ethyl 2-butyl-1,3-dithiane-2-carboxylate
-
125443-02-9
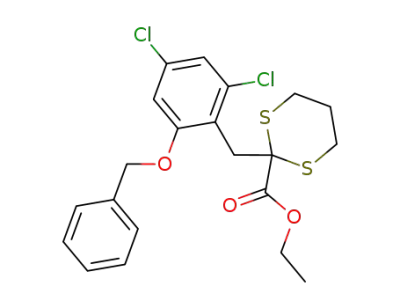
2-(2-Benzyloxy-4,6-dichloro-benzyl)-[1,3]dithiane-2-carboxylic acid ethyl ester
-
90626-79-2
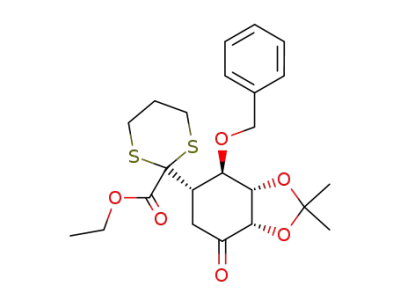
2L-(2,3,5/4)-4-O-Benzyl-5-(2-ethoxycarbonyl-1,3-dithian-2-yl)-2,3-O-isopropyliden-2,3,4-trihydroxycyclohexanon
Relevant Products
-
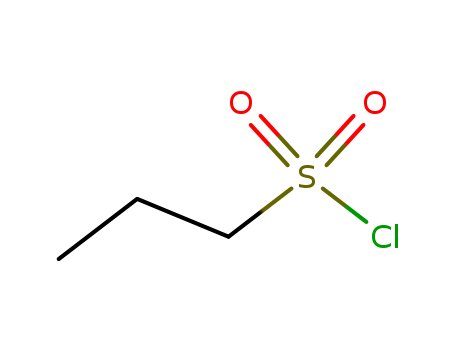
Propanesulphonyl chloride
CAS:10147-36-1
-
Benzotrifluoride
CAS:98-08-8
-
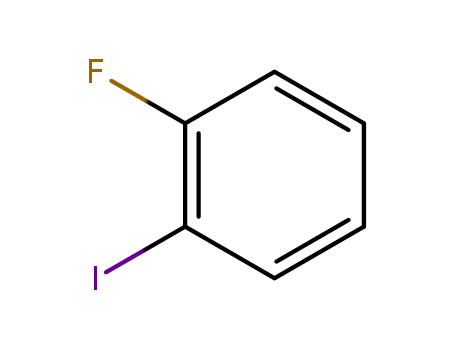
1-Fluoro-2-iodobenzene
CAS:348-52-7