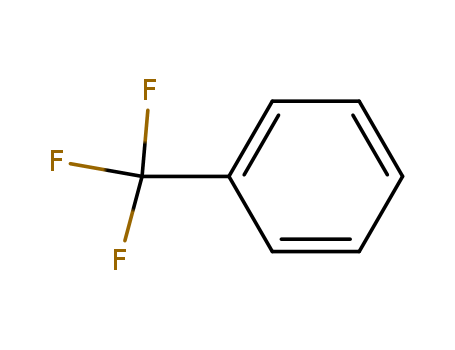
98-08-8
- Product Name:Benzotrifluoride
- Molecular Formula:C7H5F3
- Purity:99%
- Molecular Weight:146.112
Product Details;
CasNo: 98-08-8
Molecular Formula: C7H5F3
Appearance: Clear colourless to light yellow liquid
factory and supplier 98-08-8 Benzotrifluoride in stock
- Molecular Formula:C7H5F3
- Molecular Weight:146.112
- Appearance/Colour:Clear colourless to light yellow liquid
- Vapor Pressure:35.3mmHg at 25°C
- Melting Point:-29 °C(lit.)
- Refractive Index:1.414
- Boiling Point:104.769 °C at 760 mmHg
- Flash Point:12.222 °C
- PSA:0.00000
- Density:1.188 1.118 g/cm>3
- LogP:2.70540
Benzotrifluoride(Cas 98-08-8) Usage
|
Air & Water Reactions |
Highly flammable. Insoluble in water. |
|
Reactivity Profile |
Benzotrifluoride may react with oxidizing materials, strong bases and reducing agents. |
|
Hazard |
Highly toxic by inhalation. Flammable, dangerous fire risk. |
|
Fire Hazard |
HIGHLY FLAMMABLE: Will be easily ignited by heat, sparks or flames. Vapors may form explosive mixtures with air. Vapors may travel to source of ignition and flash back. Most vapors are heavier than air. They will spread along ground and collect in low or confined areas (sewers, basements, tanks). Vapor explosion hazard indoors, outdoors or in sewers. Runoff to sewer may create fire or explosion hazard. Containers may explode when heated. Many liquids are lighter than water. |
|
Flammability and Explosibility |
Notclassified |
|
Safety Profile |
Poison by intraperitoneal route.Moderately toxic by subcutaneous route. Dangerous fire hazard. To fight fire, usewater, foam, CO2, spray mist, dry chemical. When heatedto decomposition it emits toxic fumes of F??. Incompatiblewith oxidizing materials |
|
Purification Methods |
Purify benzotrifluoride by repeated treatment with boiling aqueous Na2CO3 (until no test for chloride ion is obtained), dry it with K2CO3, then with P2O5, and fractionally distil it. [Beilstein 5 IV 802.] |
|
Definition |
ChEBI: (trifluoromethyl)benzene is a fluorohydrocarbon that is fluoroform in which the hydrogen is substituted by a phenyl group. It has a role as a solvent and an environmental contaminant. It is a fluorohydrocarbon and a member of (trifluoromethyl)benzenes. It derives from a fluoroform. |
|
General Description |
A clear colorless liquid with an aromatic odor. Flash point of 54°F. Vapors are heavier than air. Insoluble in water and slightly denser than water. May be toxic by inhalation. |
InChI:InChI=1/C7H5F3/c8-7(9,10)6-4-2-1-3-5-6/h1-5H
98-08-8 Relevant articles
A NEW METHOD FOR THE TRIFLUOROMETHYLATION OF AROMATIC COMPOUNDS
Marhold, A.,Klauke, E.
, p. 516 (1980)
-
Trifluoromethylation of organic halides with methyl halodifluoroacetates - a process via difluorocarbene and trifluoromethide intermediates
Duan, Jian-Xing,Su, De-Bao,Chen, Qing-Yun
, p. 279 - 284 (1993)
Treatment of methyl halodifluoroacetates...
Aryl-CF3 Coupling from Phosphinoferrocene-Ligated Palladium(II) Complexes
Ferguson, Devin M.,Bour, James R.,Canty, Allan J.,Kampf, Jeff W.,Sanford, Melanie S.
, p. 519 - 526 (2019)
This article describes a detailed invest...
New Vistas in Transmetalation with Discrete “AgCF3” Species: Implications in Pd-Mediated Trifluoromethylation Reactions
Martínez de Salinas, Sara,Mudarra, ángel L.,Benet-Buchholz, Jordi,Parella, Teodor,Maseras, Feliu,Pérez-Temprano, Mónica H.
, p. 11895 - 11898 (2018)
This work describes the employment of di...
Application of Visible-to-UV Photon Upconversion to Photoredox Catalysis: The Activation of Aryl Bromides
Majek, Michal,Faltermeier, Uwe,Dick, Bernhard,Pérez-Ruiz, Raúl,JacobivonWangelin, Axel
, p. 15496 - 15501 (2015)
The activation of aryl-Br bonds was achi...
Photoredox-catalyzed reduction of halogenated arenes in water by amphiphilic polymeric nanoparticles
Eisenreich, Fabian,Kuster, Tom H. R.,Palmans, Anja R. A.,van Krimpen, David
supporting information, (2021/10/05)
The use of organic photoredox catalysts ...
Cross-Coupling through Ag(I)/Ag(III) Redox Manifold
Demonti, Luca,Mézailles, Nicolas,Nebra, Noel,Saffon-Merceron, Nathalie
supporting information, p. 15396 - 15405 (2021/10/12)
In ample variety of transformations, the...
Mechanistic Insight into Copper-Mediated Trifluoromethylation of Aryl Halides: The Role of CuI
Jin, Yuxuan,Leng, Xuebing,Liu, He,Shen, Qilong,Wu, Jian
, p. 14367 - 14378 (2021/09/13)
The synthesis, characterization, and rea...
H2-Free Selective Dehydroxymethylation of Primary Alcohols over Palladium Nanoparticle Catalysts
Yamaguchi, Sho,Kondo, Hiroki,Uesugi, Kohei,Sakoda, Katsumasa,Jitsukawa, Koichiro,Mitsudome, Takato,Mizugaki, Tomoo
, p. 1135 - 1139 (2020/12/29)
The dehydroxymethylation of primary alco...
98-08-8 Process route
-
-
55642-42-7
bis(trifluoromethyl)tellurium

-
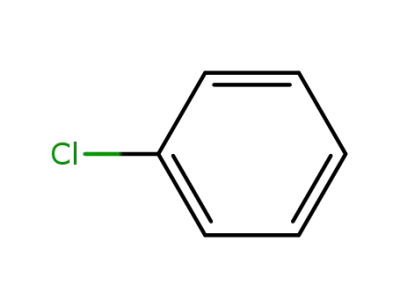
-
108-90-7
chlorobenzene

-
-
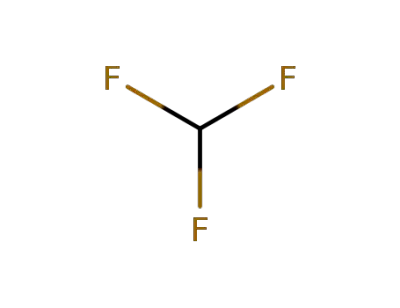
75-46-7
trifluoromethan

-
-
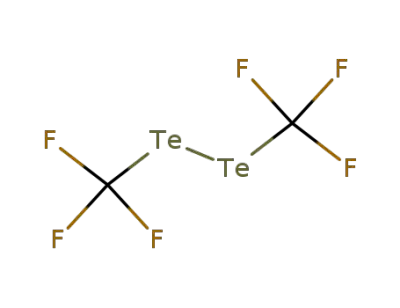
1718-20-3
bis(trifluoromethyl) ditelluride

-
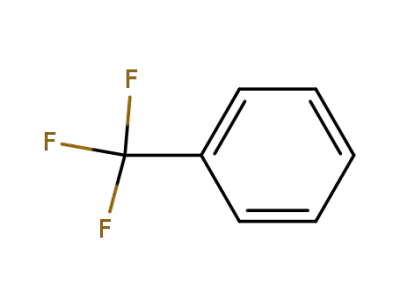
-
98-08-8
α,α,α-trifluorotoluene

-
-
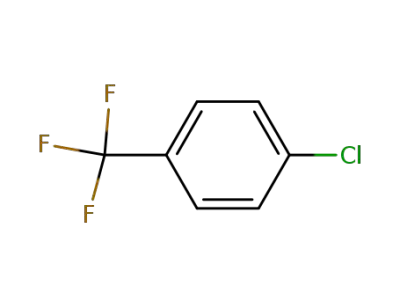
98-56-6
4-chlorobenzotrifluoride

-
-
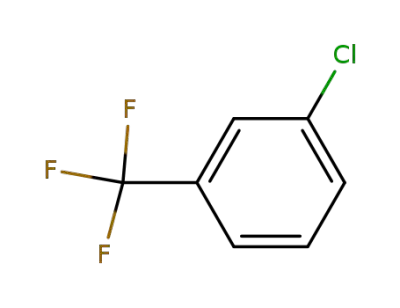
98-15-7
3-chlorotrifluoromethylbenzene

-
-
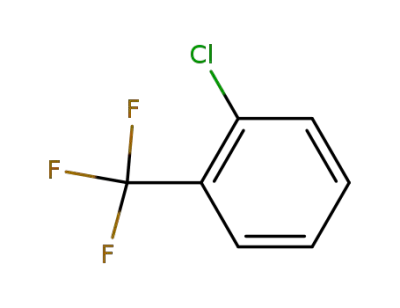
88-16-4
1-chloro-2-(trifluoromethyl)benzene
| Conditions | Yield |
|---|---|
|
at 168 ℃;
for 120h;
Product distribution;
|
-
-
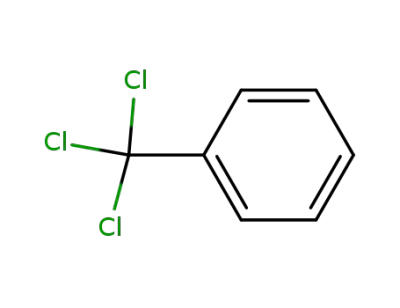
98-07-7
Benzotrichlorid

-
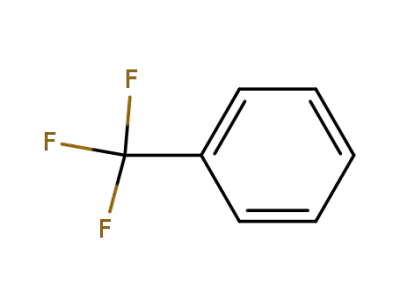
-
98-08-8
α,α,α-trifluorotoluene

-
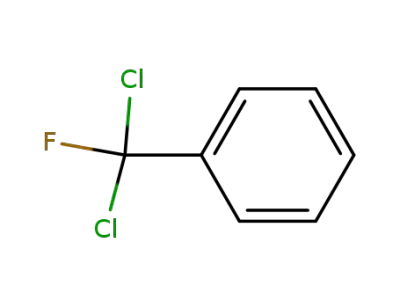
-
498-67-9
(dichlorofluoromethyl)benzene

-
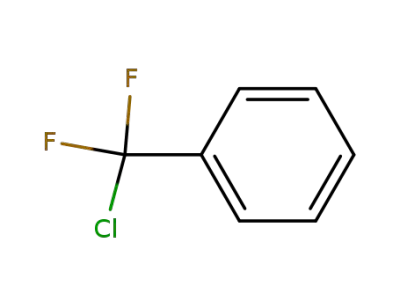
-
349-50-8
(chlorodifluoromethyl)benzene
| Conditions | Yield |
|---|---|
|
With
antimonypentachloride; pyridine hydrogenfluoride;
at 50 ℃;
for 1h;
under 7500.75 Torr;
Autoclave;
|
98-08-8 Upstream products
-
81290-20-2
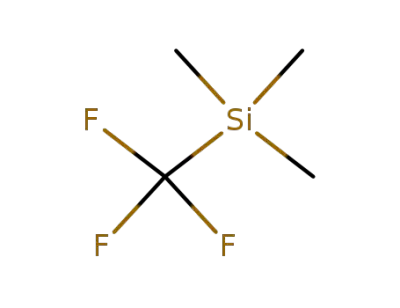
(trifluoromethyl)trimethylsilane
-
98-80-6
phenylboronic acid
-
591-50-4
iodobenzene
-
93-58-3
benzoic acid methyl ester
98-08-8 Downstream products
-
93-58-3

benzoic acid methyl ester
-
2315-68-6
propyl benzoate
-
454-92-2
3-(trifluoromethyl)benzoic acid
-
455-24-3
4-trifluoromethylbenzoic acid
Relevant Products
-
5-Bromo-2-fluoro-m-xylene
CAS:99725-44-7
-
5-Bromo-2-chlorotoluene
CAS:54932-72-8
-
Ethyl 1,3-dithiane-2-carboxylate
CAS:20462-00-4