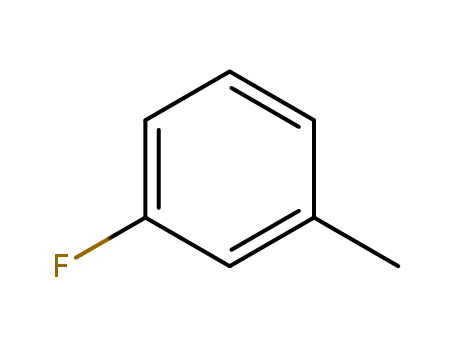
352-70-5
- Product Name:3-Fluorotoluene
- Molecular Formula:C7H7F
- Purity:99%
- Molecular Weight:110.131
Product Details;
CasNo: 352-70-5
Molecular Formula: C7H7F
Appearance: clear colorless to light yellow liquid
factory and supplier 352-70-5 3-Fluorotoluene in stock
- Molecular Formula:C7H7F
- Molecular Weight:110.131
- Appearance/Colour:clear colorless to light yellow liquid
- Vapor Pressure:20.1mmHg at 25°C
- Melting Point:-87 °C
- Refractive Index:n20/D 1.469(lit.)
- Boiling Point:118.2 °C at 760 mmHg
- Flash Point:9.4 °C
- PSA:0.00000
- Density:1.001 g/cm3
- LogP:2.13410
3-Fluorotoluene(Cas 352-70-5) Usage
|
Synthesis Reference(s) |
Journal of the American Chemical Society, 72, p. 4809, 1950 DOI: 10.1021/ja01166a506 |
|
Air & Water Reactions |
Highly flammable. |
|
Reactivity Profile |
3-Fluorotoluene may be incompatible with strong oxidizing and reducing agents. Also may be incompatible with many amines, nitrides, azo/diazo compounds, alkali metals, and epoxides. Generates toxic fluoride fumes when burned. |
|
Purification Methods |
Purify it as for o-fluorotoluene. [Beilstein 5 H 290, 5 III 676, 5 IV 799.] |
|
General Description |
3-Fluorotoluene (m-fluorotoluene) is a key aromatic compound used as a model system to study substituent effects on methyl groups attached to benzene rings. It serves as a foundational molecule in investigations of electronic interactions between substituents (e.g., halogens, OMe, SCF3, CN) and the aromatic π-system, particularly through 19F NMR analysis. The study highlights its role in elucidating how substituents influence electron density distribution, with effects ranging from additive (e.g., CN) to saturation (e.g., halogens, SCF3) upon substitution. These insights advance the understanding of through-space and inductive effects in substituted toluenes. |
InChI:InChI=1/C7H7F/c1-6-3-2-4-7(8)5-6/h2-5H,1H3
352-70-5 Relevant articles
Full continuous flow synthesis process of fluorine-containing aromatic hydrocarbon compounds
-
Paragraph 0095-0108, (2021/04/07)
The invention provides a full continuous...
Radical Decarboxylative Carbometalation of Benzoic Acids: A Solution to Aromatic Decarboxylative Fluorination
Xu, Peng,López-Rojas, Priscila,Ritter, Tobias
supporting information, p. 5349 - 5354 (2021/05/05)
Abundant aromatic carboxylic acids exist...
Pd-Co catalysts prepared from palladium-doped cobalt titanate precursors for chemoselective hydrogenation of halonitroarenes
Bustamante, Tatiana M.,Dinamarca, Robinson,Torres, Cecilia C.,Pecchi, Gina,Campos, Cristian H.
, (2019/12/24)
Bimetallic Pd-Co catalysts supported on ...
Decarbonylation of Aromatic Aldehydes and Dehalogenation of Aryl Halides Using Maghemite-Supported Palladium Catalyst
Ajda?i?, Vladimir,Nikoli?, Andrea,Simi?, Stefan,Manojlovi?, Dragan,Stojanovi?, Zoran,Nikodinovic-Runic, Jasmina,Opsenica, Igor M.
, p. 119 - 126 (2017/12/27)
A facile decarbonylation reaction of a v...
352-70-5 Process route
-
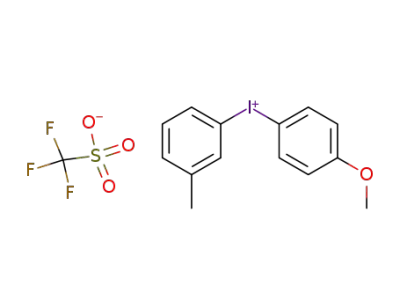
-
3-methyl-4'-methoxydiphenyliodonium triflate

-
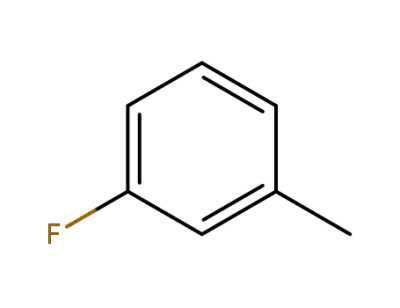
-
352-70-5
m-Fluorotoluene

-
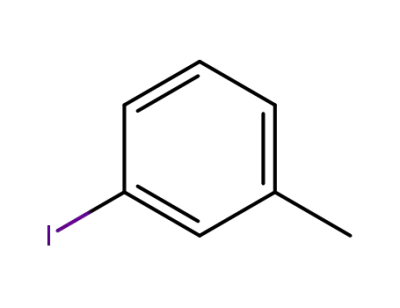
-
625-95-6
3-Iodotoluene

-
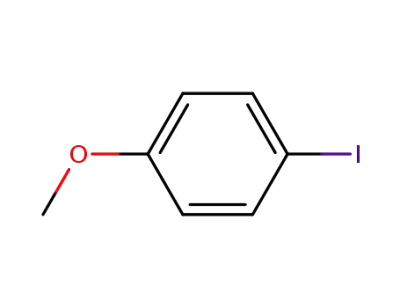
-
696-62-8
para-iodoanisole

-
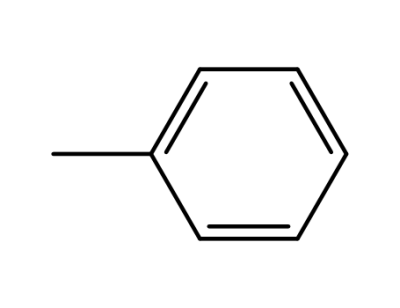
-
108-88-3,15644-74-3,16713-13-6
toluene
| Conditions | Yield |
|---|---|
|
With
cesium fluoride;
In
acetonitrile;
at 85 ℃;
for 0.666667h;
GC-MS estimated relative yields;
|
-
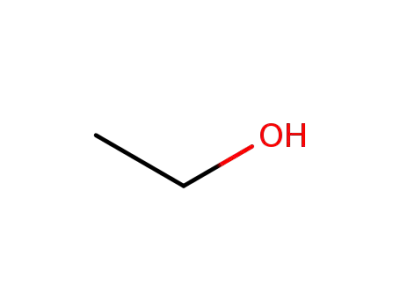
-
64-17-5
ethanol

-
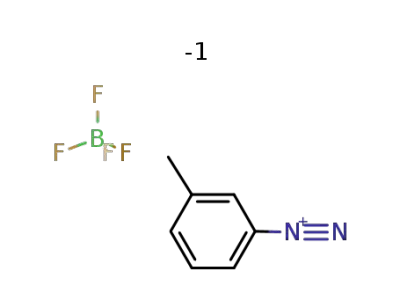
-
1422-76-0
3-methylbenzenediazonium tetrafluoroborate

-

-
352-70-5
m-Fluorotoluene

-
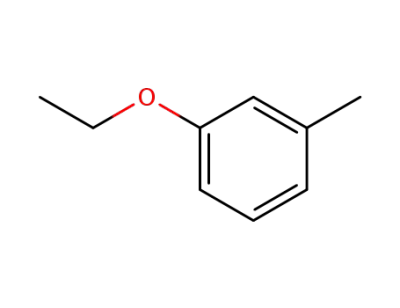
-
621-32-9
3-ethoxytoluene

-

-
108-88-3,15644-74-3,16713-13-6
toluene
| Conditions | Yield |
|---|---|
|
Heating;
|
82 % Chromat. 15 % Chromat. 3 % Chromat. |
352-70-5 Upstream products
-
108-44-1
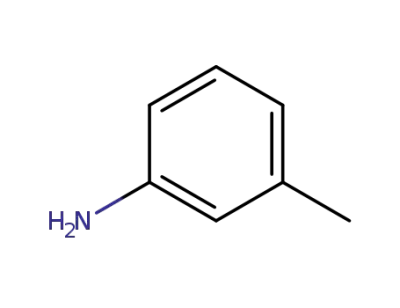
1-amino-3-methylbenzene
-
462-06-6
fluorobenzene
-
593-53-3
Methyl fluoride
-
75-45-6
Chlorodifluoromethane
352-70-5 Downstream products
-
390-04-5
2-(2-fluoro-4-methyl-benzoyl)-benzoic acid
-
328-60-9
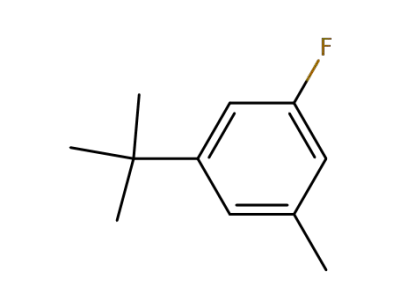
1-tert-butyl-3-fluoro-5-methyl-benzene
-
446-33-3
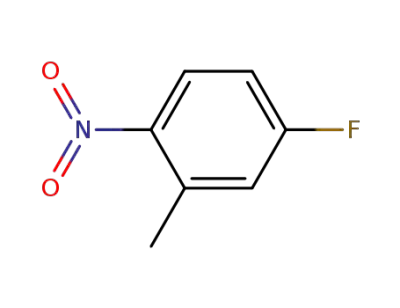
5-Fluoro-2-nitrotoluene
-
446-34-4
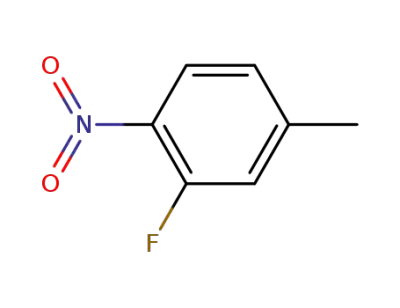
2-fluoro-4-methyl-1-nitrobenzene
Relevant Products
-
5-Bromo-2-fluoro-m-xylene
CAS:99725-44-7
-
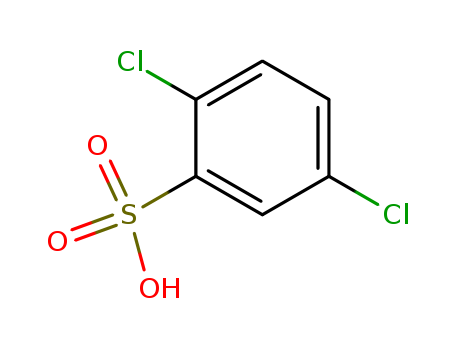
2,5-Dichlorobenzenesulphonic acid
CAS:88-42-6
-
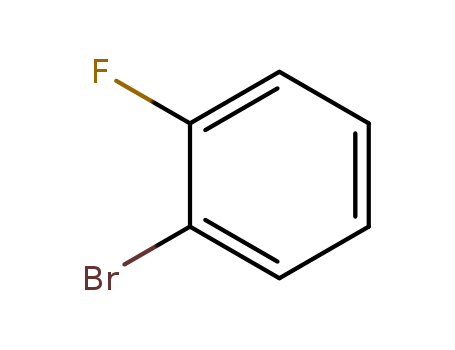
2-Bromofluorobenzene
CAS:1072-85-1